
Physiology News Magazine
Do birds experience visual illusions?
An object or motion we see does not always exist in the real world due to misinterpretations of retinal images by the brain. Where and how visual illusions occur are debated, and their elucidation would bridge psychology and neuronal activity
Features
Do birds experience visual illusions?
An object or motion we see does not always exist in the real world due to misinterpretations of retinal images by the brain. Where and how visual illusions occur are debated, and their elucidation would bridge psychology and neuronal activity
Features
Shu-Rong Wang, Yu-Qiong Niu, Rui-Feng Liu, Le-Qing Wu, & Qian Xiao
Institute of Biophysics, Chinese Academy of Sciences, Beijing, China
https://doi.org/10.36866/pn.67.27

Psychologists and physiologists hold a view that the visual illusion is subjective perception of something that is seen but lacks physical counterpart. For example, movie is actually the visual illusion of motion and continuity of a sequence of stationary photographs. Visual illusions reflect how visual information is processed and thus may act as a ‘window’ into the mysteries of the brain.
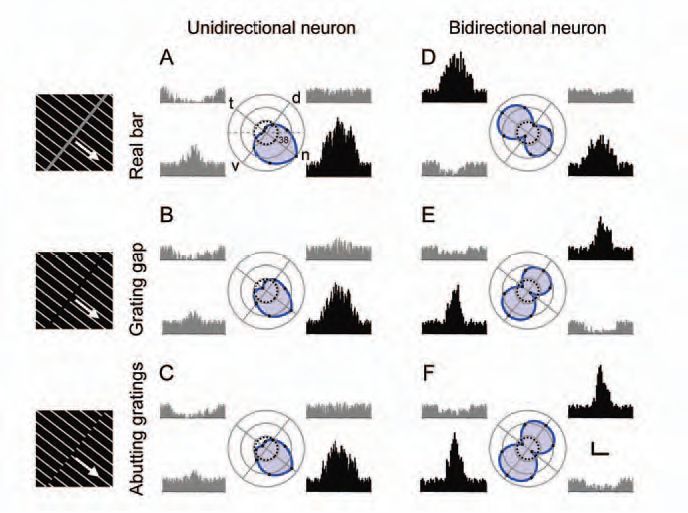
An object is segregated from background by its shape defined by contours or edges. Real contours are defined by ‘first-order’ visual cues such as luminance or colour contrasts; some other contours cannot be directly seen but inferred by the brain from ‘second-order’ cues such as textures and relative motion and thus called illusory contours. In experimental studies, a gap formed between two groups of gratings and phase-shifted abutting gratings are usually used as illusory contours because they are inferred from textures but not directly seen from luminance contrasts (Fig. 1). Motion is also a main physical attribute of an object. If you stare at running water in a waterfall for minutes and then shift your gaze to objects on the bank, you will see the objects moving upwards. This phenomenon is called the waterfall illusion or motion after-effect (MAE) in general (Anstis et al. 1998).
In recent years, the response characteristics of visual neurons to illusory contours and motion have attracted a great deal of attention (Eagleman, 2001; Nieder, 2002). It is known that cortical neurons in monkeys and telencephalic neurons in owls can detect illusory contours. On the other hand, MAE also occurs in cortical areas sensitive to visual motion. All this suggests that visual illusions may be exclusively detected in cortical neurons (Anstis et al. 1998). However, whether visual illusions are also processed in subcortical areas remains unknown and how to explain the neuronal mechanisms underlying MAE is intensely debated (Eagleman, 2001; Nieder, 2002).
The bird visual system provides a good model to study the neuronal mechanisms underlying visual illusions for two reasons. First, visual neurons in the pretectal nucleus lentiformis mesencephali (nLM) can detect real edges in gratings moving through their receptive fields (Fu et al. 1998), implying that they may also be able to detect illusory contours. On the other hand, nLM and its mammalian counterpart, the nucleus of the optic tract, are both involved in generating optokinetic nystagmus to stabilize retinal images so that their neurons should detect anything in motion. It is interesting to note that the excitatory (ERF) and inhibitory receptive field (IRF) of pretectal neurons in pigeons overlap in visual space but possess opposite directionalities (Fu et al. 1998; Cao et al. 2004). This opponent RF organization implies that after-responses of pretectal neurons to cessation of visual stimulus motion in one direction would create illusory motion in the opposite direction.

In our experiments, pretectal ERF and IRF were mapped with a computer and color-coded (Fig. 2). Real and illusory contours were generated and moved by the computer as visual stimuli presented to the pigeon, and electrophysiological responses of single nLM neurons to these stimuli were recorded with micropipettes filled with a solution containing sodium acetate for recording neuronal spikes and pontamine skyblue for dye-marking recording sites. Firing spikes in pretectal neurons were collected on-line and analyzed off-line with the computer (Niu et al. 2006).
All the 204 neurons recorded in the pigeon’s nLM responded vigorously to visual motion. They could be divided into two groups according to their selectivity for the direction of motion: one group contains 195 unidirectional neurons that fired maximal rate to motion in one (preferred) direction and minimal rate in the opposite (null) direction, and the other group includes nine bidirectional cells which were characterized by maximal rates in two opposite directions and minimal rates in the two directions orthogonal to both preferred directions.
The pretectal neurons examined in our study all responded similarly to real and illusory contours in terms of firing patterns and rates. The preferred directions in the unidirectionals were identical for real and illusory contours whereas those in the bidirectionals were changed by ~ 90 visual degrees for real versus illusory contours (Fig. 1). It seems that pretectal bidirectionals can discriminate real from illusory contours but unidirectionals cannot. On the other hand, some nLM neurons produce excitatory responses to visual motion and inhibitory after-responses to cessation of prolonged motion in the preferred directions, or inhibitory responses to visual motion and excitatory after-responses to cessation of prolonged motion in the null direction (Fig. 2). These after-responses to cessation of prolonged motion in the preferred (null) direction were similar to the visual responses to real motion in the null (preferred) direction. They had threshold duration of motion and persisted longer as motion duration was increased. These characteristics are quite similar to those of MAE reported by the subjects in our psychological experiments. Because the ERF and IRF of a pretectal cell overlap in visual space and possess opposite directionalities, after-responses to cessation of prolonged motion in one direction may create illusory motion in the opposite direction. To histologically verify the exact locations of recorded neurons, 25 recording sites were marked with dye and all were localized within the pretectal nucleus (Niu et al. 2006).
It appears that illusory contours and motion could be detected at the earliest stage of central information processing and processed in bottom-up streams from subcortical areas to the telencephalon, and that the waterfall illusion or motion aftereffect may result from functional interactions of ERF and IRF with opposite directional selectivity in visual neurons.
References
Anstis S, Verstraten FAJ & Mather G (1998). The motion aftereffect. Trends Cogn Sci 2, 111-117.
Cao P, Gu Y & Wang SR (2004). Visual neurons in the pigeon brain encode the acceleration of stimulus motion. J Neurosci 24, 7690-7698.
Eagleman DM (2001). Visual illusions and neurobiology. Nat Rev Neurosci 2, 920-926.
Fu YX, Xiao Q, Gao HF & Wang SR (1998). Stimulus features eliciting visual responses from neurons in the nucleus lentiformis mesencephali in pigeons. Vis Neurosci 15, 1079-1087.
Nieder A (2002). Seeing more than meets the eye: processing of illusory contours in animals. J Comp Physiol A 188, 249–260.
Niu YQ, Xiao Q, Liu RF, Wu LQ & Wang SR (2006). Response characteristics of the pigeon’s pretectal neurons to illusory contours and motion. J Physiol 577, 805-813.
