
Physiology News Magazine
What structures bear the tension and store energy in lengthening muscle?
When active muscles are stretched the cross-bridge cycle is truncated by suppressing the power stroke and thereby reducing ATP consumption; the high tension they exert is generated by stretch-induced strain in the pre-stroke cross-bridges. Other, noncrossbridge, structures also make a substantial contribution to tension and energy storage
Features
What structures bear the tension and store energy in lengthening muscle?
When active muscles are stretched the cross-bridge cycle is truncated by suppressing the power stroke and thereby reducing ATP consumption; the high tension they exert is generated by stretch-induced strain in the pre-stroke cross-bridges. Other, noncrossbridge, structures also make a substantial contribution to tension and energy storage
Features
Gavin Pinniger (1,2), K W Ranatunga (1), & Gerald Offer (1)
1: Muscle Contraction Group, Department of Physiology, School of Medical Sciences, University of Bristol, Bristol, BS8 1TD, UK
2: Current address is School of Biomedical and Chemical Sciences, The University of Western Australia, Crawley, WA 6009, Australia
https://doi.org/10.36866/pn.65.13

Textbooks of physiology often give the impression that when muscles are activated to produce tension, they either shorten performing work or remain at constant length if restrained by a suitable load or antagonistic muscle (isometric contraction). Yet, stretch of an active muscle, somewhat confusingly referred to as an eccentric contraction, occurs frequently during daily activities. Examples include the lengthening of the biceps when lowering a heavy weight, or of the calf muscles when walking downstairs. Lengthening contractions may also serve as a braking mechanism to decelerate the body when running downhill or landing from a jump. Lengthening muscles also store energy which may be used during a subsequent shortening contraction to enhance the tension produced; this phenomenon is the underlying basis of plyometric exercise-training. Yet our understanding of the molecular processes involved in lengthening has been unclear.

An active muscle strongly resists being stretched, developing up to twice the isometric tension. Yet the consumption of the fuel, ATP, and hence the energy output (heat plus work) declines. What is the molecular explanation for this seeming paradox? It will be recalled that the structural basis of muscle contraction is the cross-bridge cycle, the interaction of heads of the myosin molecules (M) of the thick filaments with actin (A) filaments. The Lymn-Taylor scheme summarises the main events in this cycle (Fig. 1A). Hydrolysis of ATP occurs on myosin heads detached from actin (step 1). The heads carrying the products of hydrolysis, ADP and Pi, then attach to actin (step 2). Tension is generated by a power stroke, a conformational change in the myosin head, associated with release of Pi (step 3). Detachment of post-stroke heads, after they have lost ADP and rebound ATP, completes the cycle (step 4). How does this crossbridge cycle adapt to lengthening? Since the power stroke generates force, it is strain-sensitive and hence it is expected to be slowed by the increased strain on the heads caused by the lengthening.
So a simple explanation for the reduction of ATP consumption in lengthening muscle is that the crossbridge cycle is truncated, the powerstroke and subsequent detachment of post-stroke heads being increasingly shut down as the lengthening velocity is increased. But if the power stroke rate is reduced, how do we account for the increased tension exhibited during lengthening? The pre-stroke heads that attach to actin, given a sufficient lifetime, will be dragged to higher strains and this will produce force; the heads will eventually detach when highly strained and reattach to actin subunits further away from the Z-disc (Lombardi & Piazzesi, 1990; Fig. 1B). It had been supposed that a special mechanism (forcible detachment) might operate when tension prised the prestroke heads off the actin but it is now appreciated that when force is applied to a complex of two proteins, it merely enhances the spontaneous rate of dissociation. So tension can be generated during lengthening without the power stroke, the high force being borne by pre-stroke, rather than poststroke heads (Getz et al. 1998; Pinniger et al. 2006). Our computer simulations of active muscle based on the Lymn-Taylor scheme are able to account for the lengthening limb, as well as the shortening limb, of the force-velocity relation without the need to postulate extra intermediates in the cross-bridge cycle.
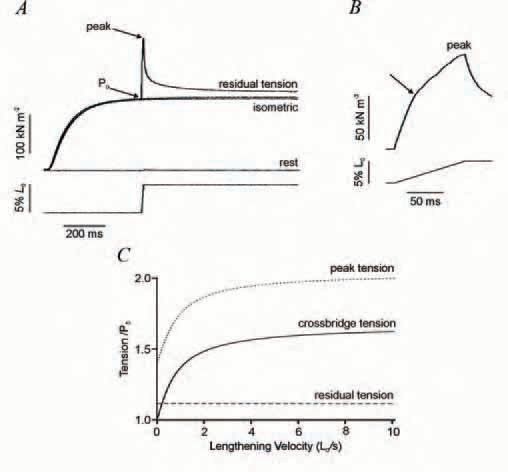
So far we have discussed only the contribution that cross-bridges make to tension. But there are strong indications that other, non-crossbridge, structures also contribute (Edman & Tsuchiya, 1996). For example, during lengthening, when work is done on the muscle, much of the energy absorption cannot be explained simply by the enhanced cross-bridge strain (Linari et al. 2003). Two further indications are shown by the tension responses during and after the application of a constant velocity stretch to a bundle of isometrically contracting muscle fibres (Fig. 2). There is an initial rapid phase of tension rise due to the cross-bridges being increasingly strained, interrupted early on by a small but detectable dip in tension as the post-stroke heads undergo the reverse power stroke. There is then a marked reduction in the rate of tension rise (Fig 2B); simulations suggest that this transition (indicated by the arrow in Fig. 2B) is due to the cross-bridges approaching the new steady-state truncated cycle (Fig. 1B). However, the tension continues to rise slowly thereafter to the end of the stretch. After the stretch, tension decays to a level which is higher than the isometric tension.
Both this continuing slow tension rise after the transition and this residual force enhancement are hard to explain on the basis of cross-bridges acting alone and have been attributed to a non-cross-bridge component. The peak tension increases with velocity of stretch to a plateau of about twice the isometric force, whereas the residual force enhancement remains the same irrespective of velocity. Figure 2C illustrates the velocity dependence of the contribution to tension by crossbridges (measured by the tension at the transition) and the contribution by noncross-bridge elements. The crossbridge tension rises steeply to a plateau 60% higher than the isometric force at high velocity, whereas the non-crossbridge tension is insensitive to velocity. Two further findings support the existence of these two tension components. Firstly, the tension decay after ramp stretch also shows fast and slow phases. Secondly, when active muscle force is drastically reduced by a specific myosin-crossbridge inhibitor (BTS), the rapid phase of tension rise during stretch and the fast phase of tension decay afterwards are greatly diminished, whereas the slow phases of tension rise and decay and the residual force remain high (Pinniger et al. 2006). Our interpretation is that noncross-bridge structural elements stiffen on activation and provide a tension that increases progressively throughout the stretch. The peak tension thus has contributions from both cross-bridge and non-crossbridge elements; the residual force enhancement arises from a partially decayed non-crossbridge force.
The residual force enhancement and continued slow tension rise during a stretch had been attributed to sarcomere inhomogeneity and sarcomere popping, uncontrolled elongation of sarcomeres. However, no convincing direct evidence of sarcomere popping has been observed experimentally and, tellingly, no popping is observed when active myofibrils are stretched even though their tension response to stretch shows the above features (Telley et al. 2006). Our proposition is that interactions between actin and the titin filaments that connect thick filaments to the Z-disc, or between actin and Cprotein (an accessory protein of thick filaments) are recruited by the release of Ca2+ on activation and play a significant role in energy storage.
Acknowledgements
We thank the Wellcome Trust for support and Hamish Roots for help with illustrations.
References
Edman KA & Tsuchiya T (1996). Strain of passive elements during force enhancement by stretch in frog muscle fibres. J Physiol 490, 191-205.
Getz EB, Cooke R & Lehman SL (1998). Phase transition in force during ramp stretches of skeletal muscle. Biophys J 75, 2971-2983.
Linari M, Woledge RC & Curtin NA (2003). Energy storage during stretch of active single fibres from frog skeletal muscle. J Physiol 548, 461-474.
Lombardi V & Piazzesi G (1990). The contractile response during steady lengthening of stimulated frog muscle fibres. J Physiol 431, 141-171.
Pinniger GJ, Ranatunga KW & Offer GW (2006). Crossbridge and non-crossbridge contributions to tension in lengthening muscle: force-induced reversal of the power stroke. J Physiol 573, 627-643.
Telley A, Stehle R, Ranatunga KW, Pfitzer G, Stüssi E & Denoth J (2006). Dynamic behaviour of half-sarcomeres during and after stretch in activated psoas myofibrils: sarcomere asymmetry but no “sarcomere popping” J Physiol 573, 173-185.
