
Physiology News Magazine
Autoregulation of neuronal activity by presynaptic GABA ARs
Presynaptic GABAA receptors have recently been identified in several cell types. Alain Marty and Sheyla Mejia-Gervacio present evidence showing that these receptors are able to shape neuronal firing patterns and discuss the role of this regulatory process for the maturation of synaptic networks in the cerebellum
Features
Autoregulation of neuronal activity by presynaptic GABA ARs
Presynaptic GABAA receptors have recently been identified in several cell types. Alain Marty and Sheyla Mejia-Gervacio present evidence showing that these receptors are able to shape neuronal firing patterns and discuss the role of this regulatory process for the maturation of synaptic networks in the cerebellum
Features
Sheyla Mejia-Gervacio & Alain Marty
Laboratoire de Physiologie Cérébrale CNRS UMR 8118, Paris, France.
https://doi.org/10.36866/pn.64.19

It has been known for decades that GABAARs exist in presynaptic terminals and in axonal locations of crustacean nerve-muscle preparations, as well as in the mammalian spinal cord. However, their exact functional role remains poorly understood. Presynaptic GABAARs presumably sense ambient GABA, which remains in the interstitial fluid after synaptic activity from neighbouring neurons. An alternative, recently explored, possibility is that GABA is released by the neuron containing the receptors. In this case, presynaptic GABAARs act as autoreceptors.
Due to the technical difficulties of recording the currents generated by activation of presynaptic GABAAR in axons, studies showing clear functional GABAAR-mediated presynaptic conductances have remained scarce. Recent examples include the suprachiasmatic nucleus, where axonal GABAARs have been proposed to be involved in the modulation of neurotransmitter release (Belenky et al. 2003). In hippocampal mossy fibers there is evidence for the co-release of GABA and glutamate after seizures (Gutierrez, 2000), and axonal GABAARs have been identified (Ruiz et al. 2003) but their functional significance remains unexplored. In the interneurons of the molecular layer of the cerebellar cortex (MLIs), Pouzat & Marty (1999) showed that, following an action potential, it is possible to record GABAAR-mediated currents which are generated in the axons. These currents could be differentiated from those of somatodendritic origin on the basis of their slower kinetics and small amplitude fluctuations. These axonal GABAAR-mediated conductances act as autoreceptors, since GABA binds back to the cell from which it was just released (Fig. 1A).
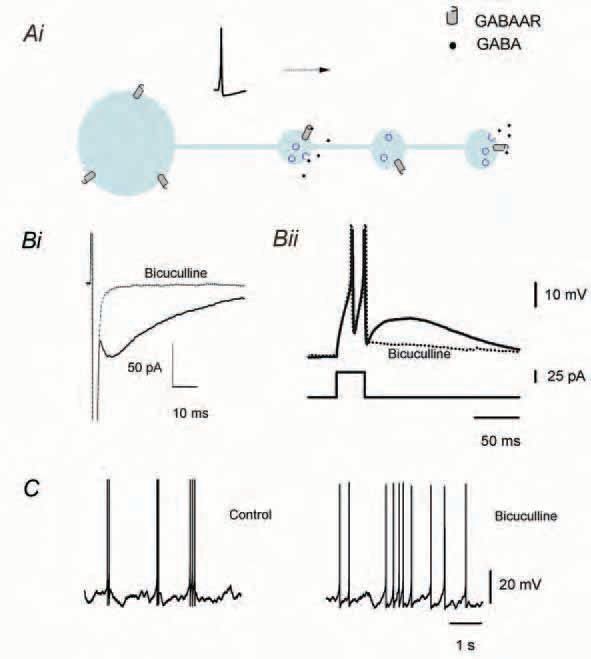
In a recent study we addressed the functional consequences of GABAA autoreceptor activity in MLIs. Our experiments showed that the activation of this axonal conductance produces a slow afterspike potential that could be recorded in the soma (Mejia-Gervacio & Marty, 2006). When using a physiological intracellular concentration of Cl-, which in MLIs is above equilibrium (15 mM: Chavas & Marty, 2003), this afterpotential is depolarizing. As shown in Fig. 1B, both the autoreceptor current (i) and the afterdepolarization (ii) are sensitive to GABAAR blockers (Pouzat & Marty, 1999; Mejia-Gervacio & Marty, 2006). The GABAA autoreceptor-mediated afterdepolarization lasts about 150 ms and its peak amplitude reaches from 5 to 20 mV. Its activation increases the presynaptic afterspike excitability, sometimes producing afterdischarge.
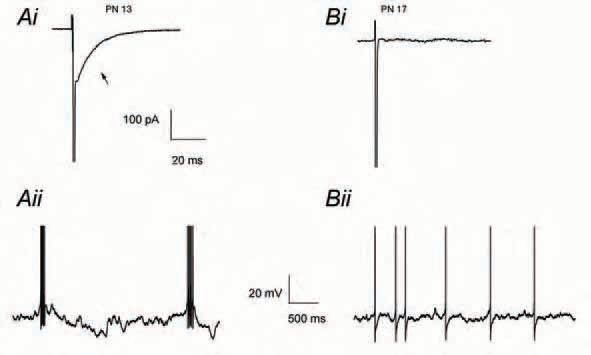
The increase in afterspike excitability, mediated by GABAA autoreceptor currents, shapes the firing pattern of MLIs, promoting discharge in short 10-20 Hz bursts. Conversely, blocking these conductances leads to a more regular firing pattern, as shown with pharmacological blockers (Fig 1C), or following washout of autoreceptor currents after long periods in whole-cell configuration (Mejia-Gervacio & Marty, 2006). Spontaneous activity in bursts was found in MLIs during the developmental stage immediately following the establishment of MLI-PC synapses, namely PN 8-15. This bursting pattern gradually fades with the GABAA autoreceptor conductance, until both phenomena disappear completely at PN 17 (Fig. 2). Thus, the expression of the autoreceptor current and its regulation of firing pattern, coincide with the period in which the synapse MLI-PC shows an immature phenotype (PN 8-15). It seems likely that the transient expression of axonal GABAAR, and possibly of other presynaptic receptors as well, influences the establishment and maturation of synaptic contacts. Specifically, repetitive presynaptic firing could help synapse stabilization by enhancing the release probability of the neurotransmitter. Moreover, the control of neuronal excitability by axonal GABAARs makes this process susceptible to modulations affecting both axonal conductance and membrane potential, since the size of the afterspike depolarization diminishes at values approaching ECl.
Presynaptic GABAARs thus seem to be involved in the autoregulation of neuronal activity. The sign of this regulation is likely cell type-specific, and will depend on factors such as ECl (see above), as well as some other unexplored ones. In periglomerular cells of the olfactory bulb, GABA is released from dendrites, and binds back to dendritic autoreceptors (Smith & Jahr, 2002). In this case, the activation of GABAARs inhibits firing, even though the position of ECl is quite depolarized in these cells, as it is in MLIs. The reason why dendritic autoreceptors of periglomerular cells are inhibitory, while the axonal autoreceptors of MLIs are excitatory, is uncertain, but may be related to the timing of GABA release, which may be more precise in the latter case.
References
Belenky MA, Sagiv N, Fritschy JM & Yarom Y (2003). Presynaptic and postsynaptic GABAA receptors in rat suprachiasmatic nucleus. Neuroscience 118, 909-923.
Chavas J & Marty A (2003). Coexistence of excitatory and inhibitory GABA synapses in the cerebellar interneuron network. J Neurosci 23, 2019-2031.
Gutierrez R (2000). Seizures induce simultaneous GABAergic and glutamatergic transmission in the dentate gyrus-CA3 system. J Neurophysiol 84, 3088-3090.
Mejia-Gervacio S & Marty A (2006). Control of interneurone firing pattern by axonal autoreceptors in the juvenile rat cerebellum. J Physiol 571, 43-55.
Pouzat C & Marty A (1999). Somatic recording of GABAergic autoreceptor current in cerebellar stellate and basket cells. J Neurosci 19, 1675-1690.
Ruiz A, Fabian-Fine R, Scott R, Walker MC, Rusakov DA & Kullmann DM (2003). GABAA receptors at hippocampal mossy fibers. Neuron 39, 961-973.
Smith TC & Jahr CE (2002). Self-inhibition of olfactory bulb neurons. Nat Neurosci 5, 760-766.
