
Physiology News Magazine
Muscle damage and exercise: does the brain contribute to muscle weakness?
Some forms of exercise lengthen contracting muscles. This damages the myofibrils when we are not accustomed to the exercise, and full recovery takes some days. Recent studies by Simon Gandevia and colleagues have looked not only at the peripheral changes in the muscle but at the changes in the central nervous system. Voluntary activation of the muscle is impaired after eccentric exercise and contributes to muscle weakness. This impairment depends on the length of the muscle
Features
Muscle damage and exercise: does the brain contribute to muscle weakness?
Some forms of exercise lengthen contracting muscles. This damages the myofibrils when we are not accustomed to the exercise, and full recovery takes some days. Recent studies by Simon Gandevia and colleagues have looked not only at the peripheral changes in the muscle but at the changes in the central nervous system. Voluntary activation of the muscle is impaired after eccentric exercise and contributes to muscle weakness. This impairment depends on the length of the muscle
Features
Trevor Allen, Jane Butler, Simon Gandevia, Orawan Prasartwuth, & Janet Taylor
Prince of Wales Medical Research Institute, Randwick, NSW, Australia
https://doi.org/10.36866/pn.64.21
Most of us have, at some time, experienced the sensation of muscle pain, stiffness and weakness the day after some unaccustomed exercise, such as running or hiking. These symptoms typically last for several days, and indicate that our muscles have been temporarily damaged. Studies into this phenomenon go back more than 100 years (Hough, 1902). The damage is greater when the exercise involves contractions in which the muscle has been actively lengthened and used as a brake or shock absorber. These are termed eccentric contractions. During exercise we use our muscles as both motors and brakes, and they are more prone to damage when used as brakes.
A characteristic property of skeletal muscle is its length-tension relation. A muscle produces its maximal isometric tension or force at a specific optimal length, and this force decreases when the muscle operates at lengths longer or shorter than the optimum. In humans when muscle is damaged after eccentric contractions, the length-tension relation and optimal length actually shifts to the right, in the direction of longer lengths (e.g. Prasartwuth et al. 2006, see Fig.1). Hence the exercise induces different degrees of weakness at different muscle lengths.

Research into exercise-induced muscle damage has largely focussed on peripheral factors responsible for the prolonged weakness, in particular the muscle fibres themselves, as morphological evidence of damage to myofibrils has been reported in humans after eccentric contractions (for review see Proske & Morgan, 2001). But what about central factors – is it possible that a reduced ability of the brain to drive the muscle contributes to the weakness? It certainly contributes to reduced maximal force in some acute bouts of fatiguing exercise (for review see Gandevia, 2001). Our recent evidence suggests that the answer is also ‘yes’ when muscle is damaged by exercise.
We tested for changes in voluntary activation of elbow flexors after a series of eccentric contractions, using twitch interpolation (Prasartwuth et al. 2005). This technique is used to estimate how effectively the muscle is being driven by the central nervous system during a maximal voluntary contraction (MVC). The amount of ‘extra’ force (if any) produced by an electrically-evoked twitch during a MVC is compared to the force produced by the same stimulus in a resting muscle. If, for example, the extra force produced by a twitch during a MVC is 10% the size of the resting twitch, voluntary activation is calculated as 90% (Gandevia, 2001).
We first found that after the elbow flexor muscles performed eccentric exercise until maximal voluntary force had fallen by ~40%, there was a significant reduction in voluntary activation by 19% (from 97 ±1% to 79 ±7%), when measured at 90° elbow angle (Prasartwuth et al. 2005). This reduction persisted for 2 days after the exercise, suggesting that the decrease was not simply due to a short-term effect such as metabolic fatigue. Eccentric muscle damage is more prominent if the muscle is lengthened from a length beyond its optimum (Morgan, 1990), but we wanted to know whether changes in voluntary activation also showed a major dependence on muscle length. As a follow-up, the study was repeated, but this time maximal voluntary force and voluntary activation were measured across a wide range of elbow angles, between 60° (short length) and 150° (long length). Again, eccentric exercise was performed until MVC force dropped by 40%.
After exercise, the optimal angle for MVC torque had shifted to the right (by ~16o). Voluntary activation was reduced significantly at all test angles in the early stages after exercise (2 hours, 1 day), and had not fully recovered after a week (being still impaired at the shortest test length of 60°). Interestingly, the size of the reduction showed a length-dependence, being more impaired at shorter muscle lengths (Fig. 2). Subjects did not report muscle pain until a day after the exercise, so that, at least initially (2 hours post-exercise), pain was not responsible for the impaired voluntary activation. An additional finding (supported by our initial study), was that the force produced by a twitch had decreased by ~80%, about twice as much as that of the voluntary contraction. This deficit in twitch force remained well below control (~50%) after 1 week.
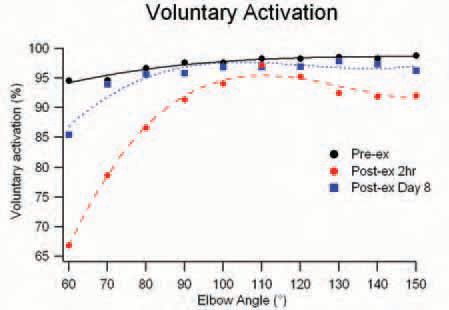
So what do these findings mean? First, the weakness after exercise was not just due to damage to the muscle, but also to a reduced ability of the brain to drive the damaged muscle, and this central contribution to weakness was more pronounced at short muscle lengths. Second, the prolonged and much greater relative force loss for twitch contractions (compared to MVCs) suggests that ‘weakness’ for more commonly performed submaximal contractions may be more pronounced than for the maximal ones. Finally, the results highlight the short- and longterm changes in the relation between the mechanical output of the muscle motor and the output of the spinal motoneurones needed to drive the motor.
It is not clear what caused the impairment in voluntary activation. It could mean that the brain reduced its drive to the damaged muscle, or that the damaged muscle changed in such a way that the brain was no longer as effective in producing force. The latter possibility is supported by the finding that voluntary activation was length dependent both before and after damage, implying that it is influenced by the muscle’s inherent length-tension properties. Muscle fibres require a higher frequency of activation to produce tetanic fusion at a short than a long length. However, the finding that voluntary activation was impaired across the full range of muscle lengths after exercise suggests that a true reduction (or change) at the level of the brain may also be occurring.
This is indeed an exciting area to investigate further. Future studies will aim to gain more insight into the interaction between central and peripheral factors limiting force output of skeletal muscles.
References
Gandevia SC (2001). Spinal and supraspinal factors in human muscle fatigue. Physiol Rev 81, 1725-1789.
Hough T (1902). Ergographic studies in muscular soreness. Am J Physiol 7, 76-92.
Morgan DL (1990). New insights into the behaviour of muscle during active lengthening. Biophys J. 57, 209-221.
Prasartwuth O, Taylor JL & Gandevia SC (2005). Maximal force, voluntary activation and muscle soreness after eccentric damage to human elbow flexor muscles. J Physiol 567, 337-348.
Prasartwuth O, Allen TJ, Butler JE, Gandevia SC & Taylor JL (2006). Length-dependent changes in voluntary activation, maximum voluntary torque and twitch responses after eccentric damage in humans. J Physiol 571, 243-252.
Proske U & Morgan DL (2001).Muscle damage from eccentric exercise: mechanism, mechanical signs, adaptation and clinical applications. J Physiol 537, 333-345.
