
Physiology News Magazine
Muscle mass at the top: a likely role for fibre hyperplasia in humans
The extreme increase in muscle mass observed after heavy resistance training cannot be simply explained on the basis of muscle fibre hypertrophy, as is commonly assumed
Features
Muscle mass at the top: a likely role for fibre hyperplasia in humans
The extreme increase in muscle mass observed after heavy resistance training cannot be simply explained on the basis of muscle fibre hypertrophy, as is commonly assumed
Features
Giuseppe D’Antona
Department of Experimental Medicine, Human Physiology Unit and Interuniversity Institute of Myology (IIM), University of Pavia, Italy
https://doi.org/10.36866/pn.63.26

The increase in skeletal muscle mass in response to several stimuli including intense physical exercise is considered an obvious example of cell plasticity. The accepted mechanism underlying such changes is quantitative modifications of gene expression.
In skeletal muscle each gene can be upregulated or downregulated in response to several factors including mechanical load (exercise), neural discharge and hormones. Gene products can be quantitatively modified and new functional and structural features can appear at macroscopic level. In particular, the ‘quantitative mechanism’ of muscle plasticity seems to be the major accepted factor by which skeletal muscle can adapt to variable functional requirements through changes in mass and fibre size (hypertrophy). Quantitative changes of fibre size are also commonly considered as the major determinants of load-induced changes in muscle force generation.

The foremost factor shaping muscle phenotype through the quantitative mechanisms is exercise resistance training (Fig. 1). Resistance training has been extensively studied and is well known to stimulate muscle hypertrophy.
Interestingly, the level of muscle mass hypertrophy described in various works appears to depend on the experimental design of the study. In fact comparative studies of different subjects populations (cross-sectional studies) have shown higher but comparable degree of muscle hypertrophy (MacDougall et al. 1982; D’Antona et al. 2006), whereas longitudinal studies of shorter (2-14 weeks) duration have shown much lower degree of hypertrophy (Aagaard et al. 2001).
It is generally assumed that the increase in muscle mass following resistance training can be fully accounted for by individual muscle fibre hypertrophy and hypertrophic response appears to be fibre-type specific. Whereas hypertrophy clearly spares slow fibres, it selectively involves fast fibres (Aagaard et al. 2001; D’Antona et al. 2006). The cause of such fibre-type selectivity is unclear and requires future investigations.
The mechanism by which heavy work increases muscle mass, through the increase of fibres size, may be the activation of protein synthesis pathways. The expression of Insulinlike Growth Factor-I (IGF-I), induced by muscle overload, and insulin have been demonstrated to be responsible for regulation of protein synthesis by stimulating the Phosphoinositide 3’Kinase/Protein Kinase (PI3K/Akt) pathway, which in turn results in the downstream activation of targets required for protein synthesis. Recently it has also been demonstrated that activation of Akt is able to induce an increase in muscle mass through a dramatic increase in fibre size of individual fibres (Glass, 2005).
Notwithstanding an undoubted role of fibres hypertrophy, the traditionally accepted quantitative mechanism of muscle plasticity is not fully able to explain the observed changes in muscle mass due to resistance exercise. In fact, studies performed on resistance athletes have shown a wide range of muscle hypertrophy without a strict correlation with changes in fibre size. This observation is most clear when considering the few available studies on body builders. These reported only limited hypertrophy of muscle fibres, failing to account for the obvious and extreme hypertrophy of whole muscles (MacDougall et al. 1982; Tesch & Larsson, 1982).
The observed discrepancy between the mean fibre cross sectional area and the anatomical cross sectional area (CSAanat, cross sectional area of a muscle determined in a plane axial to the long axis of a muscle) of the muscle determined by MRI clearly suggests that hypertrophy cannot fully explain the observed hypertrophy of whole muscle (MacDougall et al. 1982; Tesch & Larsson, 1982).
Which mechanisms underlie such discrepancy?
Which other phenomena than fibres hypertrophy contribute to the striking increase in muscle mass due to long-term and very high intense resistance training in humans?
In a recent study we aimed to help answer this question by investigating if changes in muscle architecture may explain the difference between mean fibre area and CSAanat observed in elite male body builders.
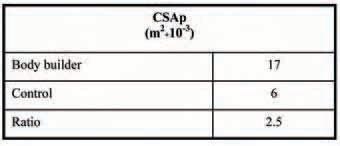
It is known that the relation between the physiological CSA (CSAphysiol, cross sectional area of a individual muscle fibres of a muscle, Table 1), and the anatomical CSA (CSAanat) is not constant. The latter relation varies according to the pennation angle, which is the angle between the axis of the fibres and the axis of the muscle (Narici et al. 1996). Physical exercise is known to induce significant adjustments of the spatial orientation of the muscle fibres. This varies the pennation angle, which has been found to increase following resistance training and to be larger in body builders than in controls (Kawakami et al. 1995). An increase in the pennation angle has thus been considered as a possible source of the discrepancy between CSAphysiol and CSAanat.
In our work, we considered known values of pennation angle and measured values of CSAphysiol and CSAanat of the vastus lateralis of elite body builders and untrained subjects. By applying these values to a simple theoretical model of muscle architecture, we excluded the idea that such differences could be entirely attributed to differences in pennation angle between the two groups of subjects (D’Antona et al. 2006).
These results combined with the observation of signs of muscle regeneration (the appearance of a low percentage of neonatal myosin isoforms) allowed us to hypothesize that hyperplasia represented the most feasible mechanism, overlapping with the increase in fibres size, leading to the observed increase in muscle mass after chronic heavy resistance training.
Unfortunately, to date, only indirect observations like ours support this hypothesis in humans. In animal models (rat or chicken) some evidence does suggest that hyperplasia can occur in overloaded conditions or after stretching.
The possible mechanisms involved in exercise-induced muscle hyperplasia are only partially known. It has been demonstrated that muscle overload induces new fibre formation resulting from satellite cell activation, proliferation, differentiation and fusion, similar to what happens during development (Tamaki et al. 1997). It is unclear to what extent satellite cell proliferation and fusion arise within the exercised muscle and the fibre injury appears the origin of the regenerative response. In both rats and chickens subjected to muscle overload there is evidence that cells formed de novo may arise in interstitial tissue between existing fibres (Fig. 2).
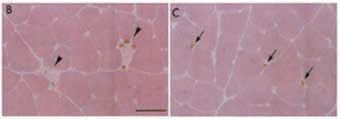
Regarding the possible signals involved in activation of quiescent satellite cells, a number of extracellular factors are known to be involved in muscle regeneration that is triggered in response to muscle injury. Some of them (Myogenic Transcription Factors, MRFs, IGF; Fibroblast Growth Factors, FGF; Hepatocyte Growth Factor, HGF; Sonic hedgehog, Shh and others) are involved in the activation of adult stem cells and in their proliferation, while others (MRFs, IGF, Neuregulin, NRG, Shh, Wingless-Int, Wnt) promote muscle differentiation. Their possible role in determining the hyperplastic response after chronic heavy resistance training deserves further investigation.
Acknowledgments
I am grateful to Marco Narici and Francesca Grisù Caliaro for useful discussions on muscle architecture and regeneration. This work has been supported by a grant from Italian Space Agency (ASI) and World Anti-Doping Agency (WADA).
References
Aagaard P, Andersen JL, Dyhre-Poulsen P, Leffers AM, Wagner A, Magnusson SP, Halkjaer-Kristensen J & Simonsen EB (2001). A mechanism for increased contractile strength of human pennate muscle in response to strength training: changes in muscle architecture. J Physiol 534, 613-623.
D’Antona G, Lanfranconi F, Pellegrino MA, Brocca L, Adami R, Rossi R, Moro G, Miotti D, Canepari M & Bottinelli R (2006). Skeletal muscle hypertrophy and structure and function of skeletal muscle fibres in male body builders. J Physiol 570, 611-627.
Glass DJ (2005). Skeletal muscle hypertrophy and atrophy signaling pathways. Int J Biochem Cell Biol 37, 1974-1984.
Kawakami Y, Abe T, Kuno SY & Fukunaga T (1995). Traininginduced changes in muscle architecture and specific tension. Eur J Appl Physiol Occup Physiol 72, 37-43.
MacDougall JD, Sale DG, Elder GC & Sutton JR (1982). Muscle ultrastructural characteristics of elite powerlifters and bodybuilders. Eur J Appl Physiol Occup Physiol 48, 117-126.
Narici MV, Binzoni T, Hiltbrand E, Fasel J, Terrier F & Cerretelli P (1996). In vivo human gastrocnemius architecture with changing joint angle at rest and during graded isometric contraction. J Physiol 496, 287-297.
Tamaki T, Akatsuka A, Tokunaga M, Ishige K, Uchiyama S & Shiraishi T (1997). Morphological and biochemical evidence of muscle hyperplasia following weight-lifting exercise in rats. Am J Physiol 273, C246-256.
Tesch PA & Larsson L (1982). Muscle hypertrophy in bodybuilders. Eur J Appl Physiol Occup Physiol 49, 301-306.
