
Physiology News Magazine
Interplay between neutrophils and skeletal muscle after exercise. What’s going on?
Neutrophils appear in skeletal muscle after exercise. The mechanisms by which skeletal muscle activates neutrophils and the function of neutrophils in skeletal muscle are, write Francis Pizza and colleagues, beginning to be revealed
Features
Interplay between neutrophils and skeletal muscle after exercise. What’s going on?
Neutrophils appear in skeletal muscle after exercise. The mechanisms by which skeletal muscle activates neutrophils and the function of neutrophils in skeletal muscle are, write Francis Pizza and colleagues, beginning to be revealed
Features
Francis X Pizza (1), Jennifer M Peterson (1), Joel H Baas (1), & Timothy J Koh (2)
1: Department of Kinesiology, The University of Toledo, Toledo, OH, USA
2: School of Kinesiology, University of Illinois, Chicago, IL, USA
https://doi.org/10.36866/pn.61.32


Exercise causes subpopulations of leukocytes, namely neutrophils and macrophages, to accumulate in skeletal muscle. Interestingly, neutrophils appear in skeletal muscle in the hours to days after both non-injurious exercise (e.g. stretching) and exerciseinduced muscle injury (e.g. lifting weights for the first time) (Best et al. 1999; Pizza et al. 2002; McLoughlin et al. 2003a). Little is known about how exercise orchestrates neutrophil responses (e.g. migration, reactive oxygen species (ROS) production, and release of cytokines) and how these responses influence the physiology and plasticity of skeletal muscle.
We propose that the microenvironment of skeletal muscle after exercise dictates when and how many neutrophils appear in skeletal muscle, which products they produce, and their function in skeletal muscle (Fig. 1). In our working model, exercise causes molecular, cellular, and tissue changes in skeletal muscle that take place on an injury continuum. At the beginning of the continuum, no overt signs of muscle injury (functional impairment and histological disruptions) are apparent; whereas moderate to severe injury produces overt injury. In the different regions of the continuum there are overlapping as well as discrete cues that cause neutrophils to have different functions in injured and non-injured skeletal muscle after exercise.
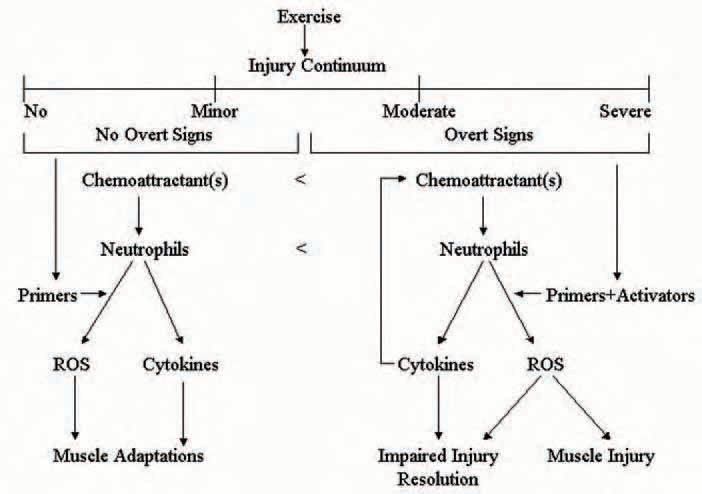
Although skeletal muscle contains several cell types (e.g. skeletal muscle cells, endothelial cells, fibroblasts, and resident macrophages) that are capable of producing factors that influence neutrophil responses, evidence from our laboratory indicates that skeletal muscle cells are an important source factors that promote neutrophil migration (chemotaxis) after exercise (Tsivitse et al. 2005). Our experiments have demonstrated that neutrophil chemotaxis in vitro and muscle neutrophil concentrations in vivo are greater after injurious relative to noninjurious exercise (Pizza et al. 2002; Tsivitse et al. 2005). Thus, injured skeletal muscle cells may release a greater concentration of neutrophil chemoattractants or a different set of chemoattractants relative to non-injured skeletal muscle cells after exercise.
Neutrophil chemotaxis after injury could be enhanced by the release of chemotactic cytokines from neutrophils (e.g. ELR + CXC chemokines) that have invaded the injured muscle.
Once neutrophils have entered injured skeletal muscle they are thought to contribute to the removal of damaged or necrotic tissue via phagocytosis. A prerequisite for phagocytosis, however, is that neutrophils encounter factors in the milieu of injured muscle that triggers the production and release of the weaponry responsible for degrading damaged or necrotic tissue (e.g. ROS production and release of proteases). Findings from our laboratory indicate that the soluble environment of injured skeletal muscle cells contains factors that both enhance (i.e. primers) and that directly increase (i.e. activators) ROS production from neutrophils (Tsivitse et al. 2005). A negative consequence of neutrophil-derived ROS is that their release into the extracellular fluid may cause ‘collateral damage’ (secondary injury) by damaging healthy regions of injured fibres and/or adjacent uninjured fibres (Fig. 2). We have provided support for this contention by demonstrating that neutrophils cause muscle dysfunction, histological abnormalities, and oxidative modification to skeletal muscle proteins in vivo after injurious exercise and by demonstrating the neutrophils injure cultured skeletal muscle cells via mechanisms that are dependent on neutrophil adhesion and ROS production (Pizza et al. 2001; McLoughlin et al. 2003b; Pizza et al. 2005).

We speculated that neutrophil-mediated injury was a necessary consequence for restoring structure and function to injured skeletal muscle. We thought it might be analogous to a home improvement project where structurally sound aspects of a construction site are often removed/damaged to optimize the restoration process. To our surprise, we found that neutrophils impaired some of the events associated with restoring structure and function to injured skeletal muscle (Pizza et al. 2005). The mechanism by which neutrophils impaired the resolution of the injury remains to be determined. Neutrophils may impair the resolution of exerciseinduced muscle injury by damaging developing skeletal muscle cells (myoblasts and/or myotubes) (Pizza et al. 2001; McLoughlin et al. 2003b) and/or by releasing cytokines (e.g. TGF-β1, TNF-α, IFN-γ, and IL-1β) that inhibit the growth of skeletal muscle cells and/or that promote skeletal muscle protein catabolism (Hawke & Garry, 2001).
Following non-injurious exercise we found that the microenvironment of skeletal muscle cells after non-injurious exercise also contain primers, but not activators, for neutrophil-derived ROS. Because non-injured muscle presumably does not contain targets for phagocytosis, primed neutrophils in non-injured muscle after exercise may release low levels of ROS. If true, then low concentrations of ROS released from neutrophils in non-injured muscle may contribute to exercise-induced skeletal muscle adaptations by influencing redox-sensitive genes. Furthermore, because neutrophils can release cytokines in the absence of phagocytosis, neutrophil-derived cytokines may serve as cellular signals for muscle adaptations after exercise. Such adaptations may include, protection from subsequent injury, muscle growth (hypertrophy), and angiogenesis.
Further work is required to test the central elements of our working model and to reveal the mechanisms for the complex interplay between neutrophils in skeletal muscle after exercise.
Once mechanisms for the interplay have been exposed, therapeutic and/or pharmacological strategies could be developed to manipulate the inflammation biology of skeletal muscle to minimize any negative consequences of neutrophils in skeletal muscle.
References
Best TM, Fiebig R, Corr DT, Brickson S & Ji L (1999). Free radical activity, antioxidant enzyme, and glutathione changes with muscle stretch injury in rabbits. J Appl Physiol 87, 74-82.
Hawke TJ & Garry DJ (2001). Myogenic satellite cells: physiology to molecular biology. J Appl Physiol 91, 534-551.
McLoughlin TJ, Mylona E, Hornberger TA, Esser KA & Pizza FX (2003a). Inflammatory cells in rat skeletal muscle are elevated after electrically stimulated contractions. J Appl Physiol 94, 876-882.
McLoughlin TJ, Tsivitse SK, Edwards JA, Aiken BA & Pizza FX (2003b). Deferoxamine reduces and nitric oxide synthase inhibition increases neutrophil-mediated myotube injury. Cell Tissue Res 313, 313-319.
Pizza FX, Koh TJ, McGregor SJ & Brooks SV (2002). Muscle inflammatory cells after passive stretches, isometric contractions, and lengthening contractions. J Appl Physiol 92, 1873-1878.
Pizza FX, McLoughlin TJ, McGregor SJ, Calomeni, EP & Gunning, WT (2001). Neutrophils injure cultured skeletal myotubes. Am J Physiol Cell Physiol 281, C335-341.
Pizza FX, Peterson JM, Baas JH & Koh TJ (2005). Neutrophils contribute to muscle injury and impair its resolution after lengthening contractions in mice. J Physiol 562, 899-913.
Tsivitse SK, Mylona E, Peterson JM, Gunning WT & Pizza FX (2005). Mechanical loading and injury induce human myotubes to release neutrophil chemoattractants. Am J Physiol Cell Physiol 288, C721-729.
