
Physiology News Magazine
Control of oxygen delivery within skeletal muscle
The delivery of O2 to contracting myocytes ultimately limits the scope of oxidative metabolism and is also an important determinant of substrate selection. New data provide insight into the matching of O2 supply and demand in muscles of differing fibre type
Features
Control of oxygen delivery within skeletal muscle
The delivery of O2 to contracting myocytes ultimately limits the scope of oxidative metabolism and is also an important determinant of substrate selection. New data provide insight into the matching of O2 supply and demand in muscles of differing fibre type
Features
Paul McDonough, Brad J Behnke, Danielle J Padilla, Timothy I Musch, & David C Poole
Pulmonary and Critical Care Medicine, University of Texas Southwestern Medical Center Dallas, TX, USA
https://doi.org/10.36866/pn.60.23

Sustained muscular activity requires a continuous and adequate O2 supply during which blood flow ( &Q) may increase more than one order of magnitude. In general, convective O2 transport ( &QO2), the product of Q and O2 concentration, increases in proportion to the elevated metabolic rate (V& O2) (reviewed by Poole, 1997). However, within the exercising muscles themselves, little is known about the matching of O2 and O2. This information is crucial to understanding muscle energetics and function during exercise, because the matching of &Q O2 and V& O2 determines the pressure of O2 within the microvasculature (PmvO2) which drives O2 diffusion from the blood into the myocyte for consumption by the mitochondria (Fig. 1). In addition, PmvO2 helps regulate intracellular PO2 which determines the cellular energetic status (Wilson et al. 1977; Hogan et al. 1992; Richardson et al. 1995). Across the spectrum of muscular activities which require varying degrees of muscular power, speed or endurance, there is a differential and specific recruitment of muscle fibre types. Muscle fibres have traditionally been differentiated based upon contraction speed and metabolic capacity (i.e. slow-twitch, highly oxidative (Type I), fast-twitch, oxidative/glycolytic (Type IIA), and highly glycolytic (Type IIB/X)). What is not universally appreciated is that these fibre types also have markedly different &QO2 responses. Specifically, highly oxidative fibres (Type I and IIA) receive the greatest &QO2 , while glycolytic fibres receive a relatively parsimonious &Q O2 (Armstrong & Laughlin, 1983).

Recently, novel phosphorescence quenching techniques have facilitated investigation of the relationship between QO2 and VAO2 across the spectrum of fibre types (Behnke et al. 2003; McDonough et al. 2005). At rest and during muscle contractions, slow-twitch soleus muscle regulates its QO2/VO2 ratio at a far higher value than its fast-twitch counterparts (medial and superficial gastrocnemius) (Fig. 2). As a consequence of this behaviour, the soleus muscle sustains a substantially higher PmvO2, thus facilitating high rates of O2 transport within the fibres of this muscle. Moreover, if that elevated PmvO2 regulates intracellular O2 levels above those found in fast-twitch muscle (fibres) it will facilitate an improved cellular energy state during contractions (↑ [ATP]/[ADP][Pi], with associated improvements in [PCr]/[Cr] and redox state; Wilson et al. 1977; Hogan et al. 1992). This effect will be independent of the oxidative or glycolytic capacity per se and will mandate faster V& O2 kinetics at the onset of contractions coupled with a decreased consumption of finite intramuscular glycogen stores and improved contractile function in the fibres of the soleus (Jones & Poole, 2005).
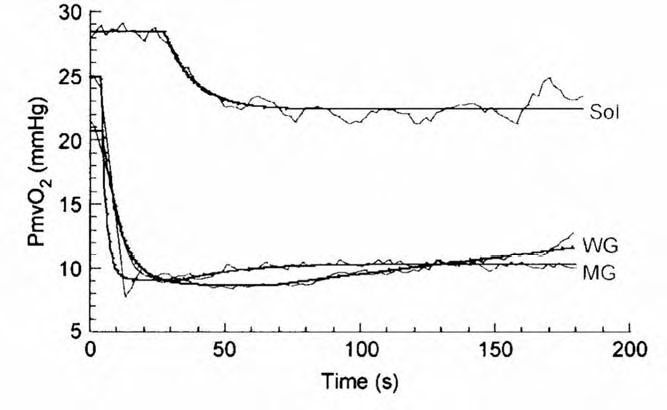
There is compelling evidence that arteriolar vasodilator capacity is lower in fast-twitch muscle via several mechanisms, which include a decreased endothelial nitric oxide synthase activity, decreased sensitivity to endothelial vasodilation and increased sensitivity to noradrenaline (Delp, 1999; Delp et al. 2000). This is the most probable cause of the lowered PmvO2 in fast- versus slow-twitch muscles. Mathematically, the reduced QO2/VO2 ratio (i.e. less PmvO2) in fast-twitch muscle could arise from an elevated VO2. However, this is not the case as VO2 is lower both at rest and during contractions in fast versus slow-twitch muscle (Behnke et al. 2003; McDonough et al. 2005).
Conclusions
There is a differential QO2 -VO2 matching and thus PmvO2 in slow- and fast-twitch muscle that is responsible, in part, for their metabolically diverse response to contractions (i.e. oxidative versus glycolytic energy production, substrate selection,VO2 kinetics and maximal VO2 ). This might constitute one mechanism by which ageing and diseases such as heart failure and diabetes (which may alter the muscle fibre type and/or impair endothelial function) will reduce PmvO2, thereby impairing diffusive blood-myocyte O2 transport and placing a low limit on exercise tolerance. Therapeutic interventions need to be developed that target preservation or restoration of arteriolar endothelial function. This strategy would elevate PmvO2 at rest and during exercise and improve mobility and health outcomes in these diseases.
References
Armstrong RB & Laughlin MH (1983). Blood flows within and among rat muscles as a function of time during high speed treadmill exercise. J Physiol 344, 189-208.
Behnke BJ, McDonough P, Padilla DJ, Musch TI & Poole DC (2003). Oxygen exchange profile in muscles of contrasting fibre types. J Physiol 549, 597-605.
Delp MD (1999). Myogenic and vasoconstrictor responsiveness of skeletal muscles is diminished by hindlimb unloading. J Appl Physiol 86, 1178-1184.
Delp MD, Colleran PN, Wilkerson MK, McCurdy MR & Muller-Delp J (2000). Structural and functional remodeling of skeletal muscle microvasculature is induced by simulated microgravity. Am J Physiol 278, H1866-H1873.
Hogan MC, Arthur PG, Bebout DE, Hochachka PW & Wagner PD (1992). Role of O2 in regulating tissue respiration in dog muscle 1992). Role of O2 in regulating tissue respiration in dog muscle working in situ. J Appl Physiol 73, 728-736.
