
Physiology News Magazine
How do receptor-associated proteins regulate the turnover of receptors at a synapse?
Features
How do receptor-associated proteins regulate the turnover of receptors at a synapse?
Features
Othon L Gervásio & William D Phillips
Department of Physiology, Institute for Biomedical Research, The University of Sydney, Sydney, Australia
https://doi.org/10.36866/pn.60.24

The packing of acetylcholine receptors (AChRs) into a post-synaptic AChR cluster ensures efficient synaptic transmission at the neuromuscular synapse. The AChR cluster is formed during embryonic development in response to signalling pathways initiated when neural agrin (released from the motor nerve) activates the Muscle Specific Kinase, MuSK (Sanes & Lichtman, 2001). A protein called rapsyn that binds the cytoplasmic domains of the AChR is then thought to cross-link AChRs and attach them to the cytoskeleton (Fig. 1). While rapsyn is essential for forming AChR clusters, it is not clear how MuSK activation initiates rapsyn-mediated AChR clustering (Sanes & Lichtman, 2001).

Humans with subtle mutations in the rapsyn coding sequence are born with AChR clusters but their post-synaptic AChR density is impaired (Ohno et al. 2002). How then does wild-type rapsyn contribute to the postnatal maturation of healthy synapses?
Life-cycle of a receptor
We tagged rapsyn with jellyfish green fluorescent protein and introduced the chimeric protein (rapsyn-EGFP) into the muscle fibres of adult mice by plasmid electroporation (Gervásio & Phillips, 2005). We used fluorescence microscopy to track the newly synthesized rapsyn-EGFP as it assembled with AChRs. Rapsyn-EGFP accumulated in the Golgi apparatus. The Golgi seems to be a staging post where rapsyn first assembles with newly synthesized AChRs on their way to the plasma membrane (Moransard et al. 2003). Fluorescence Recovery After Photobleaching (FRAP) experiments suggested that, after reaching the plasma membrane, the AChR-rapsyn complex may diffuse about in the perisynaptic membrane before it becomes entrapped in the AChR cluster (Fig. 1). Eventually AChRs escape from the AChR cluster, are internalized and are degraded in the lysosome. The half-life for survival of AChR at the synapse increases during development from about 1 day (before birth) to about 10 days in the adult rodent. Each AChR channel may form a stable (life-long) partnership with a single molecule of rapsyn (Fig. 1) (LaRochelle & Froehner, 1986).
Rapsyn-AChR stoichiometry and AChR stabilization
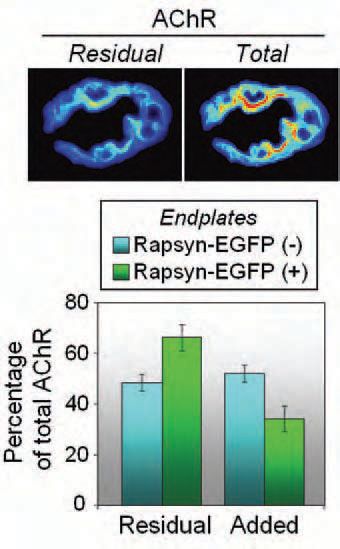
However, quantitative analysis suggests that the rapsyn-AChR relationship is not as monogamous as it once appeared (Moransard et al. 2003). Rapsyn-EGFP was able to target directly to the adult synapse, bypassing the Golgi apparatus. This nearly doubled the amount of rapsyn associated with each AChR channel. Importantly, this increase in rapsyn-AChR stoichiometry resulted in a slowing-down of the metabolic turnover of the post-synaptic AChR (Gervásio & Phillips, 2005). Over a four-day period more of the old AChR was retained and fewer newly synthesized AChRs were added to the synapse (Fig. 2). We propose that the additional rapsyn molecules stabilize the AChRs by linking them more tightly to the cytoskeleton.
Implications
Changes in rapsyn-AChR stoichiometry of a similar magnitude occur during the normal development and aging of synapses. Conceivably then, developmental increases in rapsyn-AChR stoichiometry might cause the postnatal slowing of AChR turnover at the synapse. In models of autoimmune myasthenia gravis, auto-antibodies bind AChRs, causing accelerated AChR degradation and impaired synaptic transmission. Increasing the level of rapsyn expression in the cell might offer a way of slowing this AChR loss (Phillips et al. 1997; De Baets et al. 2003). All this highlights the need to sort out the mechanisms that control rapsyn-AChR stoichiometry at the synapse.
Muscle electrical activity, and neural agrin, are two signals from the nerve terminal that can help maintain the ongoing slow AChR turnover characteristic of adult synapses (Bezakova et al. 2001). The mechanism by which they slow AChR turnover is not known, but perhaps it is by increasing rapsyn-AChR stoichiometry at the synapse.
Acknowledgements
This work was supported by a project grant from the NH&MRC, Australia and by a Sesqui R&D grant.
References
Bezakova G, Rabben I, Sefland I, Fumagalli G & Lomo T (2001). Neural agrin controls acetylcholine receptor stability in skeletal muscle fibers. Proc Natl Acad Sci (USA) 98, 9924-9929.
De Baets M, Stassen M, Losen M, Zhang X & Machiels B (2003). Immunoregulation in experimental autoimmune myasthenia gravisabout T-cells, antibodies and endplates. Annals NY Acad Sci 998, 308-317.
Gervásio OL & Phillips WD (2005). Increased ratio of rapsyn to ACh receptor stabilizes postsynaptic receptors at the mouse neuromuscular synapse. J Physiol 562.3, 673-685.
Larochelle WJ & Froehner SC (1986). Determination of the tissue distributions and relative concentrations of the postsynaptic 43-kDa protein and the acetylcholine receptor in Torpedo. J Biol Chem 261, 5270-5274.
Moransard M, Borges LS, Willmann R, Marangi PA, Brenner HR, Ferns MJ & Fuhrer C (2003). Agrin regulates rapsyn interaction with surface AChRs which underlies cytoskeletal anchoring and clustering. J Biol Chem 278, 7350-7359.
Ohno K, Engel AG, Shen X-M, Selcen D, Brengman J, Harper CM, Tsujino A & Milone M (2002). Rapsyn mutations in humans cause endplate acetylcholine-receptor deficiency and myasthenic syndrome. Am J Hum Genet 70, 875-885.
Phillips WD, Vladeta D, Han H & Noakes PG (1997). Rapsyn and agrin slow the metabolic degradation of the acetylcholine receptor. Mol Cell Neurosci 10, 16-26.
Sanes JR & Lichtman JW (2001). Induction, assembly, maturation and maintenance of a postsynaptic apparatus. Nat Rev Neurosci 2, 791-805.
