
Physiology News Magazine
Different polyamine concentrations underlie the regional difference in the strong inward rectifier K+ current in the heart
The inward rectification of a K+ current in the cardiac atrial myocytes is stronger than that in the ventricular myocytes. This new study reveals that different intracellular polyamine concentrations underlie the difference
Features
Different polyamine concentrations underlie the regional difference in the strong inward rectifier K+ current in the heart
The inward rectification of a K+ current in the cardiac atrial myocytes is stronger than that in the ventricular myocytes. This new study reveals that different intracellular polyamine concentrations underlie the difference
Features
Ding-Hong Yan & Keiko Ishihara
Department of Physiology, Faculty of Medicine, Saga University, Saga, Japan

https://doi.org/10.36866/pn.60.26
In the heart, the working myocytes of the atria and ventricles are both specialised for contraction, yet show different action potential configurations. Action potential duration is shorter in atrial than in ventricular myocytes due to the steeper slope of the plateau phase (phase 2). On the other hand, the final repolarization (phase 3) is much slower in atrial myocytes due to the smaller amplitude of their strong inward rectifier potassium current (IK1). Our new study strongly suggests that a difference in the concentrations of the intracellular polyamines that regulate the amplitude of IK1 contributes to the difference between atrial and ventricular IK1 in the guinea-pig heart (Yan et al. 2005).
The outward current through the strong inward rectifier K+ channels is much smaller than the inward current because intracellular polyamines (spermine and spermidine) block the channels in a voltage-dependent manner. Nevertheless, under physiological conditions the outward current plays the major role, as the inward current does not usually flow at physiological voltages. In working cardiac myocytes it repolarizes the cell membrane during action potentials.
Previous studies have suggested that the amplitude of the outward IK1 is smaller in the atria than in ventricles not only because of a difference in current density reflecting a difference in the number of channels in the cell membrane, but also because of the stronger rectification of the atrial current – that is, the ratio of the amplitudes of the outward and inward currents is smaller in the atria. We confirmed this in guinea-pig hearts using the amphotericin B perforatedpatch method, which minimizes changes in the intracellular polyamine concentration during patch-clamp recordings. Moreover, we found that the transient component of the outward IK1, which is the result of competition between spermine and Mg2+ (a weaker blocker of outward IK1 than polyamines) to block the IK1 channel (Ishihara & Ehara, 1998; Yan & Ishihara, 2005), is only seen in ventricular myocytes. These findings raise two possibilities: either the concentration of polyamines is higher in atrial myocytes, or the atrial IK1 channels are more susceptible to blockade by polyamines.
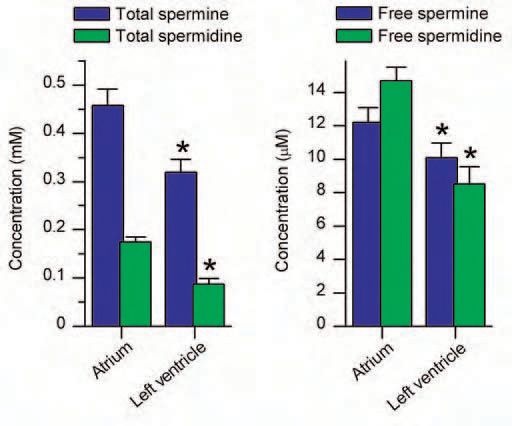
The strong inward rectifier potassium channels underlying the cardiac IK1 are considered to be formed by homo- or heterotetrameric co-assembly of three closely-related subunits: Kir2.1, Kir2.2 and Kir2.3 (Liu et al. 2001). We have determined, based on its electrophysiological properties, that in the guinea-pig heart the contribution of the Kir2.3 subunit to the IK1 channels is very minor in both atrial and ventricular myocytes. When we tested whether the difference in the sensitivities of the channels formed by either the Kir2.1 or Kir2.2 subunit to polyamines could account for the observed difference in the outward IK1 in atrial and ventricular myocytes, we found this not to be the case. As one might have expected, however, increases in the polyamine concentration could change Kir2.1 currents from the ‘ventricular type’ to the ‘atrial type’ (Fig. 1). Moreover, when we then measured the polyamine contents of cardiac muscles and estimated the concentrations of total and free polyamines, we found that they were indeed higher in the atria than in the ventricles of guinea-pig hearts (Fig. 2).
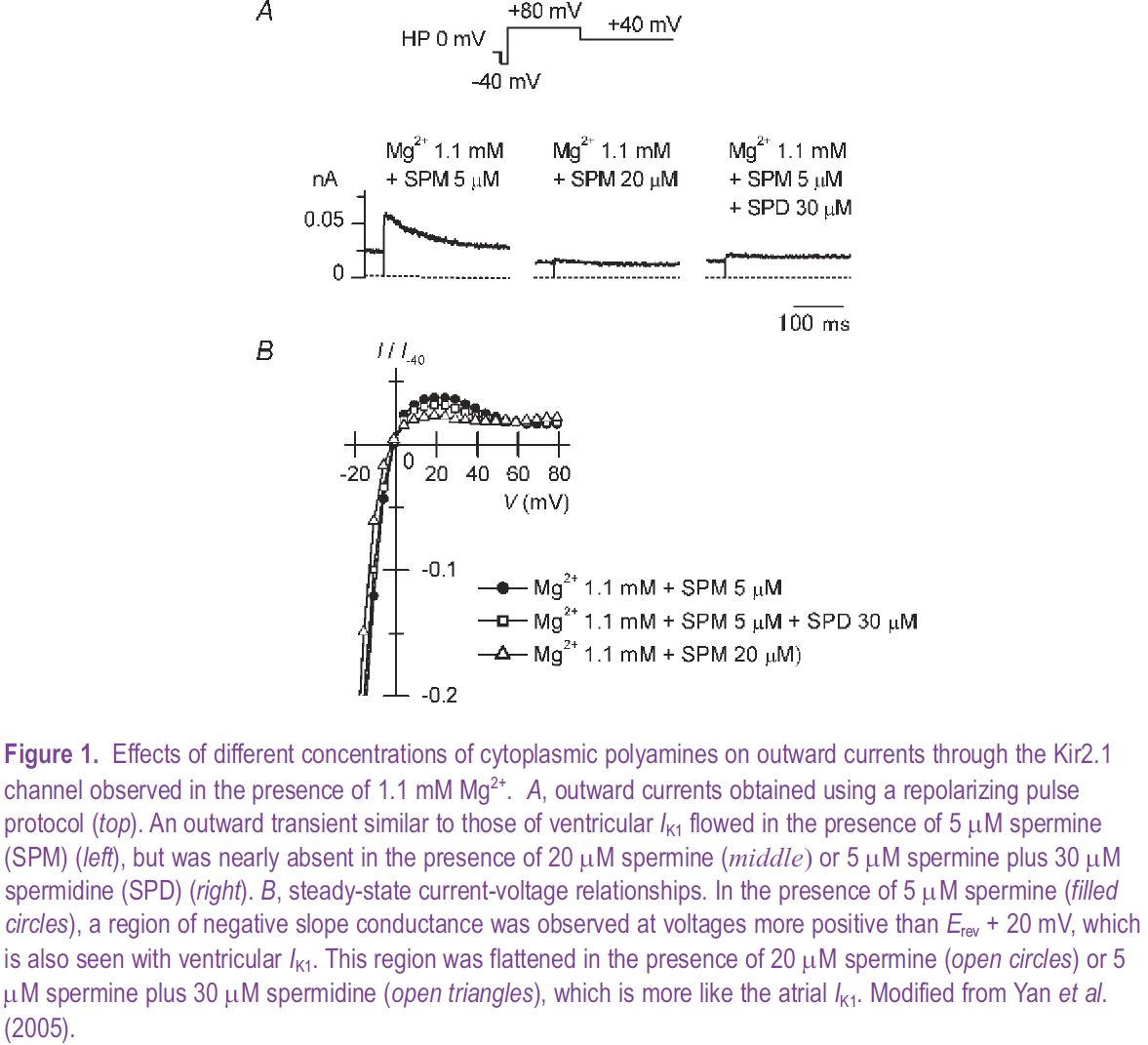
Polyamines are widely distributed in eukaryotic cells, playing important roles in cell growth and differentiation, though their levels vary among different cell types (Watanabe et al. 1991). Our study revealed that a difference in polyamine levels contributes to the regional difference in IK1 in the heart. In the heart muscle, the synthesis of polyamines and their levels within myocytes are affected by a variety of physiological and pathological factors. It is known, for example, that polyamine levels are increased during cardiac hypertrophy, which may account for the observed reduction of IK1 that accompanies prolongation of the action potentials and the resultant increased susceptibility to arrhythmia. Thus, by regulating IK1, polyamines play important roles in regulating cardiac function.
References
Yan D-H, Nishimura K, Yoshida K, Nakahira K, Ehara T, Igarashi K & Ishihara K (2005). Different intracellular polyamine concentrations underlie the difference in the inward rectifier K+ currents in atria and ventricles of the guinea-pig heart. J Physiol 563, 713-724.
Ishihara K & Ehara T (1998). A repolarization-induced transient increase in the outward current of the inward rectifier K+ channel in guinea-pig cardiac myocytes. J Physiol 510, 755-771.
Yan D-H & Ishihara K (2005). Two Kir2.1 channel populations with different sensitivities to Mg2+ and polyamine block: a model for the cardiac strong inward rectifier K+ channel. J Physiol 563, 725-744.
Liu GX, Derst C, Schlichthörl G, Heinen S, Seebohm G, Brüggemann A, Kummer W, Veh RW, Daut J & Preisig-Müller R (2001). Comparison of cloned Kir2 channels with native inward rectifier K+ channels from guinea-pig cardiomyocytes. J Physiol 532, 115-126.
Watanabe S, Kusama-Eguchi K, Kobayashi H & Igarashi K (1991). Estimation of polyamine binding to macromolecules and ATP in Bovine lymphocytes and rat liver. J Biol Chem 266, 20803-20809.
