Questions from the front line
Our COVID-19 Advisory Panel seeks to provide an evolving understanding of the physiological and pathophysiological mechanisms underpinning this disease. Read questions our COVID-19 Advisory Panel received from front line clinicians dealing with patients.

Please note that while our experts are providing advice on research data we are not able to provide medical or individual clinical advice.
Questions
Please see below for questions we have been submitted. If you are a physiologist who can contribute to the answers of any of these questions please join the forum using the link on the relevant question. To access the forum you will need to register here.
1: Abnormal coagulation [RESPONDED]
Question 1: Abnormal coagulation is a major factor here. Thromboelastography is highly coagulopathic; fibrinogen is usually high; D-Dimers are often in the multiple 100ks; platelets fall a bit. We see chest X-rays consistent with pulmonary emboli. Pulmonary arterial branch microthrombi are reported at post mortem.
a. What is driving this?
b. How does this relate to the renal failure we see?
c. How does this relate to the gas exchange problems we see?
Response: [11/04/20] In addition to the alveolitis, the patients seem to develop intense endothelial activation and thrombus formation. Endothelial activation is seen in patients with ARDS, but the magnitude of endothelial activation appears quite marked in COVID 19, which is probably triggering the thrombus formation. Small thrombi typically tend to undergo lysis quite rapidly, contributing to these very elevated d-dimers. D-dimers are a pretty good indicator for clot turnover.
High fibrinogen is an acute phase response and is similar to the elevated CRP seen in the cohort. High fibrinogen, creates more substrate that can be activated for the formation of a fibrin rich clot, which can be dense.
Alveolar inflammation is known to upregulate PAI-1 expression within the alveoli and in the peripheral circulation. It is not known how high the levels are in this group of patients. Huang et al., (Lancet, Feb 2020, 15 -21) have demonstrated that ICU patients overall had more inflammation compared to non-ICU patients.
It appears the gas exchange problems and renal problems may be related to mismatch at the peripheral level due to small vessel thrombosis. Wang and colleagues have demonstrated (prepublication Journal of haemostasis and thrombosis) an improvement in P/F ratio within a few hours of IV infusion of tissue plasminogen activator. Unfortunately, this did not translate to improved outcomes, but this rapid improvement probably reflects restoration of blood flow. We need to watch this space.
There is also considerable debate about the value of anticoagulation in this group of patients. Some clinicians are now anticoagulating their patients with therapeutic doses of low molecular weight heparin in the context of high d-dimers. Please see paper for additional information: IV TPA In COVID ARDS
2: Renal failure and ventilation [RESPONDED]
Question 2: Renal failure is unusually common and only seems to happen when a patient is mechanically ventilated.
a. What is the mechanism of vascular injury? Thrombotic/other?
Response: 11/04/2020
There is emerging evidence that delaying intubation and mechanical ventilation for as long as possible seems best given the high mortality once on mechanical ventilation. It seems that the lungs are highly compliant during a “silent hypoxaemia phase” typical of many patients and lung damage is being caused by too high inflation pressures and too high positive end expiratory pressures (PEEP). This may also reduce pulmonary blood flow from high intra-pulmonary pressures but also reduce venous return and lower right heart output.
Based on intelligence from Dr Weingart, an intesivit from New York and input from colleagues in Italy he is using the following strategies in the hypoxaemia group:
(i). High flow nasal oxygen (100%) delivery;
(ii). Use of a surgical mask for partial re-breathing to raise PaCO2
(iii) CPAP including full head immersion type with neck collar again with O2 flow which provides some PEEP
(iv) Careful control of body fluids -“running them dry” is quoted
(v) Proning; this might also help venous return and increase right heart output
Once on a ventilator, coagulation including disseminated intravascular coagulation occurs as well as a “cytokine storm” that leads to end organ damage. The following are being trialled:
Heparin
Favipiravir: antiviral drug (Japan)
Remdesivir: antiviral drug (NIH RCT)
Sarilumab, IL-6 receptor ab (RCT)
Investigational vaccine called mRNA-1273 (NIAID/ NIH trial)
Response [11/04/2020}
With regards to the renal failure, it is true that the UK are seeing a very large amount of acute kidney injury in ventilated patients. The average across England and Wales is 18.5% of patients on ICU (ICNARC data) but in the London hospitals at the heart of the outbreak the number is closer to 35-50%.
This is multifactorial but the main reasons are :
- Patients were being “run dry,” i.e. deliberately dehydrated on ICUs early on in the outbreak. This is because this is how ARDS is usually managed, to minimise interstitial oedema in the lungs. However, this has resulted in a big incidence in AKI and we have realised that this COVID19 is not a typical ARDS picture, at least at the early phase of ventilation. This practice has now change and patients are being manage euvolaemic, which will hopefully reduce the AKI rate.
- As the previous post has suggested, the cytokine storm phase of the disease can impact any organ and the kidneys are often vulnerable. In addition, these patients have been found to be quite hypercoagulable with high D-Dimers, leading some to suggest there may be a microvascular element to the AKI. This may be true but as we are not performing renal biopsies on these patients we have no evidence of this. The limited post mortum data from China and Italy has not shown any evidence of thrombotic microangiopathy.
- It appears that Black and South Asians patients are more susceptible to getting severe disease. These patients are also more likely to have the co-morbidities associated with severe disease, such as diabetes and hypertension. These conditions are also risk factors for chronic kidney disease and AKI is more likely to occur in patients with underlying CKD.
3: Intubation and hypoxaemia [RESPONDED]
Question 3: Patients, once intubated, are usually profoundly hypoxaemic. CO2 clearance is terrible. This fits with substantial VQ mismatch and a massive physiological deadspace. We need to characterise, find out why and what to do about it
a. Is this true?
b. What is the cause and what can we do about it?
Is there an acquired haemoglobinopathy?
Response: [10/04/20] The latest I have seen (Webinar from New York intensivist Dr Scott Weingart ; htps://emcrit.org/emcrit/avoiding-intubation-and-initial-ventilation-of-covid19-patients/) is that the disease is heterogenious. He describes 3 phenotypes – Silent Hypoxaemia; Indolent and Hyper acute.
I consider the silent hypoxaemic group characterised by very low oxygen saturations (and at this time are eucapnic or hypocapnic) but do not seem to develop an adequate ventilatory response, although they are able to increase ventilation volitionally. They may include rapid (40-50 bpm) shallow breathers with heart rates around 80 bts/min
A message that comes across from Dr Weingart and his colleagues in Italy LOUD & CLEAR is to delay intubation and mechanical ventilation for as long as possible. It seems the highly compliant lungs (at this stage) are being damaged by too high inflation pressures and too high positive end expiratory pressures (PEEP). This may also reduce pulmonary blood flow from high intra-pulmonary pressures but also reduce venous return and lower right heart output
The silent hypoxaemia group The New York intensivist is using
(i). High flow nasal oxygen (100%) delivery;
(ii). Use of a surgical mask for partial re-breathing to raise PaCO2
(iii) CPAP including full head immersion type with neck collar again with O2 flow which provides some PEEP
(iv) Careful control of body fluids -“running them dry” is quoted
(v) Proning; this might also help venous return and increase right heart output
Increasing respiratory drive may also assist because peripheral chemoreceptors may be targeted by COVID-19. There is evidence of pneumonia viruses down regulating HIF, which is essential for oxygen sensing in the carotid body. Incidentally, down regulation of HIF has been shown to enhance viral replication…. Also, COVID-19 infects peripheral neruones which might include petrosal ganglion which would explain (i) dysgeusia; (ii) loss of chemical drive
To enhance respiratory drive the following have been used:
(i) Add CO2 to the inspired air to prevent ‘hypocapnic braking’ and drive central and peripheral chemoreceptors;
(ii) Doxapram (Dopram, Stimulex or Respiram) – been used to resolve apnoeas of prematurity and weaning off ventilators; activates peripheral and central chemos and can be infused i.v. Not without side effects if dose is too high – well documented though. Avoid preparations with benzyl alcohol, which cause adverse effects.
(iii) Benzolamide (better than acetalzolamide). Enhances arterial CO2 will directly drive peripheral and central chemoreceptors; The effect on renal carbonic anhydrase will remove the hypocapnic braking effect on carotid body. The elevated PaCO2 will help brain blood flow.
(iv). Funcke et al. Curr Med Res Opin. 1974;2:37-42; doi: 10.1185/03007997409111739), used cyclandelate and reported improved brain blood flow/oxygenation and, importantly, glucose uptake. This could also be considered alone or with Benzolamide to drive respiratory function…..
(v). Theophylline / caffeine, – low doses. Has added advantages of bronchodilator and anti-inflammatory and PDE inhibitor.
(iv) Others – aminophylline, methylxamthines, almitrine bismesylate
Response [11/04/20]
Regarding the dreadful gas exchange observed in so many patients, we read comments such as ‘CO2 clearance is terrible’ and there being a ‘big physiological dead space’, with the word ‘shunt’ appearing frequently too. I think it is helpful when trying to understand the nature of the ventilation-perfusion mismatch that is occurring in respiratory failure not to forget two basic axioms:
1. Dead space on its own causes PaO2 to fall only to the approximate extent to which PaCO2 rises. (The relation becomes exact with an FIO2 = 1). It follows that profound hypoxaemia can only very rarely be attributed to a ‘big physiological dead space’.
2. In the presence of a pure 50% shunt, arterial gases take on the values of mixed venous gases when healthy (given no change in cardiac output, FIO2, ventilation and oxygen consumption). This is a precise axiom that is not widely appreciated but makes a useful teaching point for the definition of severe respiratory failure (i.e. failure bad enough to turn arterial blood into mixed venous blood).
These two simple almost undergraduate teaching points can help us avoid mis-attributing the complex abnormality of gas exchange in Covid-19. My colleagues at the clinical battle-front lead me to believe that what they are seeing is close to the 50% shunt.
Response [11/04/20]
Re hypoxaemia: see paper below
It is long and detailed but entirely theoretical. The take-home message seems simple – for a given haemoglobin concentration O2 carrying capacity can be markedly reduced by COVID and this may be antagonised by some treatments.
As far as I can tell no one has looked to see if the oxygen dissociation curve is shifted by COVID as predicted in this theoretical paper.
I have contacted people to see if this could be tested – but had no answer. Most clinical chemistry labs will have small volume machines for measuring dissociation curves. Surely this is something that should be done – if only to rule it out?
4: Children and women [RESPONDED]
Question 4: The strongest biological signal is that children are almost universally spared, and women highly protected. Why?
a. The CV binds to the ACE2 molecule as its receptor. Is ACE2 expression different by age or gender?
b. Is oestrogen protective?
Response [10/04/2020]
ACE2 which is involved in the binding and entry of Covid-19 is reported to be high in alveolar cells and lung parenchyma, in airway cells, cardiovascular, renal tubular cells and vasculature. Although, ACE2 is not the only requirement for Covid 19 entry, the pattern of expression is consistent with tissues that are targeted by the infection (Jai, HP et al 2005, ).
a.There is little evidence that the abundance of ACE2 (thus increased binding site for the virus) is increased in vulnerable groups. What evidence there is, indicates the opposite, that ACE is higher in women than men, and is higher in the young (Chen, J et al 2020, Gupta, M et al 2012). There is evidence that oestrogen upregulates ACE2, but this appears to be tissue specific (Brosnihan,KB et al 2008, Gupte, M 2012). Reduced estrogens and androgens could contribute to the reduction in ACE2 abundance with age. There are reported polymorphisms in ACE2 receptors that are also linked with lung injury (although analysis of a low number of samples from China did not find any indications that these contributed to severity of disease).
b.The actions mediated by ACE2 are vasodilatory and antifibrotic. The presence of ACE2 (endogenous or exogenous) was shown to protect mice from acid or sepsis induced acute lung injury (Imai, Y et al 2005). Loss of ACE2 resulted in increased vascular permeability, lung oedema, neutrophil accumulation and reduced lung function. Binding of Corona viruses to ACE2 causes shedding of ACE2 from the cell and ACE2 down regulation by the SARS Corona virus worsened lung injury. This suggests Corona viruses may exacerbate lung injury by decreasing ACE2. There is some evidence that treatment with estrogen was protective against SARS Corona virus in mice and that it elevated ACE2 in mouse kidney, adipocytes and in myocarial tissue from elderly men (Channappana, R et al 2017, Bukowska A et al 2017). Unfortunately, there is little available data to know if it is protective in people.
5: Hypertension and diabetes risk factors [RESPONDED]
Question 5: Why are people with hypertension and diabetes more at risk? Is it via ACE2 (polymorphisms are associated with hypertension).
Response: There are two relevant issues here around the association of susceptibility and more sever outcome from COVID-19 and:
1. Hypertension
2. Antihypertensives – Angiotensin Converting Enzyme inhibitors (ACEi) and Angiotensin receptor blockers (ARBs)
1. COVID-19 & Hypertension
The current dogma is that having hypertension increases vulnerability to COVID-19 and its severity. This was based on publications in Lancet Respiratory Medicine and BMJ:
Fang L, Karakiulakis G, Roth M. (2020). Are patients with hypertension and diabetes mellitus at increased risk for COVID-19 infection? Lancet Respir Med. 2020 Mar 11; doi: 10.1016/S2213-2600(20)30116-8. [Epub ahead of print]
Sommerstein R. Re: Preventing a covid-19 pandemic: ACE inhibitors as a potential risk factor for fatal Covid-19. BMJ 2020;368:m810.
However, a conclusion cannot be drawn that hypertension results in severe infection as these analyses were unadjusted for confounders such as age.
Since these publications, other publications have emerged and are consistent with the viewpoint that people with hypertension are not at increased risk from COVID-19:
% with htn Number in study Reference
15 1099 Guan et al (2020) NEJM
15 41 Huang et al (2020) Lancet
31 138 Wang et al (2020) JAMA
30 140 Zhou et al (2020) Lancet
19 201 Wu et al. (2020) JAMA
Caveats:
The numbers with hypertension reflects the numbers expected to have raised blood pressure within any population whether on antihypertensives or not.
Its unclear when the blood pressure was measured; COVID-19 can increase arterial pressure
2. COVID-19 and ARBs and ACEi
There has been an unprecedented interest around the interaction of Coronavirus (SARS-CoV2) and ACEi and ARBs and whether these medications increase the risk of COVID-19 as hypothesised by Fang et al. (2020).
Response – evidence is inconclusive and advice from learned societies and colleges globally is to maintain prescribed ACEi & ARBs for all diseases.
Angiotensin converting enzyme 2 is a membrane bound enzyme that allows coronavirus into cells. Its prevalent in type II alveolar cells that secrete surfactant, myocytes and the brainstem. Two animal studies (Ocaranza et al 2006; Ishiyama et al 2004) indicate that RAS blockers increase tissue ACE2 but other studies report no change (Burrell et al. 2005; Burchill et al. 2012). In humans, plasma ACE2 was not altered by ARBs (Walters et al. 2017).
In fact, there is evidence to support the contrary that ARBs may provide protection against ACE2 mediated entry of COVID into cells:
(i). Angiotensin 1 receptor (AT1R) antagonism with losartan prevented the internalization/degradation and ubiquitination of ACE2 (Deshotels et al. 2015)
(ii). Lower intubation rates and mortality occurred in patients with viral induced pneumonia on ACEi (and statins) (Henry et al. 2018).
So, can ARB/ACEi use (and potentially increased plasma ACE2 as a viral decoy) be beneficial in coronavirus and other viral pneumonias? Maybe.
Note trials below:
Recombinant human ACE2; pilot RCT with 24 patients and Losartan RCT in COVID-19; University of Minnesota.
Response [19/04/2020]:
Zhang P et al. Association of inpatient use of angiotensin converting enzyme inhibitors and angiotensin II receptor blockers with mortality among patients with hypertension hospitalized with COVID-19. Circ Res. 2020
indicates that ACEi and ARBs have reduced the mortality in patients versus those on non-ACEi/ARB anti-hypertensive drugs. There are caveats such as this being a retrospective study but the data are otherwise encouraging. We know that 2 prospective trials are underway looking at effects of ARBs and ACEi in COVID-19 patients that will address the issues around retrospective studies.
The accompanying commentary by Murthy et al. (2020). Circ. Res entitled: ACEing COVID-19: A Role For Angiotensin Axis Inhibition in SARS-CoV-2 infection?
makes the statement that ACEi and ARBs may be providing a protective role in COVID-19 patients.
Another paper reports the same findings (reduced mortality)
Bean et al. 2020 (MedRXiv) based on London patients: Treatment with ACE-inhibitors is associated with less severe disease with SARS-Covid-19 infection in a multi-site UK acute Hospital Trust
Our commentary published earlier this month (Talreja et al. 2020: N Z Med J. 2020 Apr 3;133(1512):85-87. A consensus statement on the use of angiotensin receptor blockers and angiotensin converting enzyme inhibitors in relation to COVID-19), we like many other global societies and foundations, made the conclusion that patients on ACEi and ARBs should remain on them despite the earlier “hypothesis” that ACE-I/ARB therapy may facilitate SARS-CoV-2 entry and increase severity of COVID-19 (Fang et al. 2020. Lancet Respir Med. 2020;8:e21). In stark contrast we also proposed that ACEi/ARBs could provide protection against SARS-CoV-2 from the following mechanisms:
1. evidence that ACEi might confer protection in some viral pneumonias (Henry C, et al. (2018). Impact of angiotensin-converting enzyme inhibitors and statins on viral pneumonia. Proc (Bayl Univ Med Cent). 31:419-423.
2. Since ACE2 can be hijacked by SARS-CoV-2 , Angiotensin II levels can rise so blocking it production and activity will be beneficial (e.g. reduce inflammation, vasoconstriction). Also, by reducing AngII, the interaction between AT1 receptors and ACE2 is increased – this might prevent viral-ACE2 coupling.
3. ARBs are known to prevent ACE2 down regulation AND internalisation so could block viral entry, although this remains untested as far as I can tell.
4. ARBs prevent viral-ACE-2 interactions so another way in which infection can be curtailed.
Conclusion: ACEi and ARBs may offer protection by reducing infectivity as well as reducing the known adverse effects (pro-inflammatory, elevated oxidative stress, vasoconstriction).
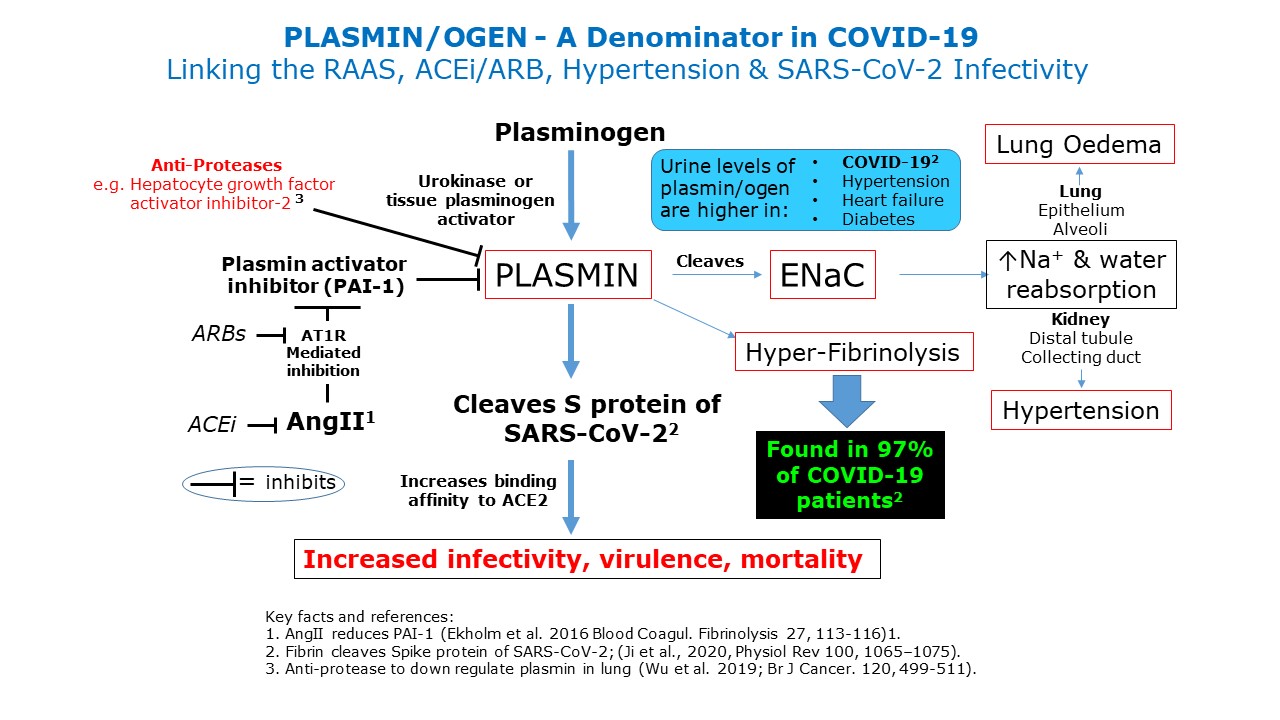
Further update [26/04/20]
I have tried to synthesise the RAAS, ACEi and ARBs, hypertension, clotting factors and susceptibility to SARS-CoV-19.
See Fig below which is explained by the following text:
Emerging evidence now suggests that vulnerable COVID-19 patients often have:
Hypertension
Diabetes
Coronary heart disease
Cerebrovascular illness
Chronic obstructive pulmonary disease
Kidney dysfunction
These patients have worse clinical outcomes when infected with SARS-CoV-2 and have raised levels of plamin(ogen) in urine.
Remarkably 97% of COVID-19 patients have elevated plasmin(ogen)
Plasmin appears to be a major protease that may cleave SARS-CoV-2 extracellularly that causes increased infectivity and virulence via ACE2.
Note that Angiotensin II can stimulate production of plasmin via inhibition of the plasmin activator inhibitor-1. Given this, blockade of Angiotensin II production (via ACEi) and AT1 receptors (via ARBs) would provide some protection against Plasmin production.
The plasmin(ogen) system may prove a promising therapeutic target for combating COVID-19. Recently, effective protease reducing plasmin activity in lung is Hepatocyte growth factor activator inhibitor-2 (Wu et al. 2019). This approach offers a potential novel therapeutic strategy.
Related links:
6: Pulmonary Artery pressures [RESPONDED]
Question 6: Pulmonary Artery pressures in most are NOT elevated as far as we can tell… but it may change with disease course. Does it?
This is an intriguing observation; the question implies that such normal PA pressure has been observed in patients who have disease severe enough to require ICU care with or without mechanical ventilation.
Response [13/04/2020]:
This is an intriguing observation; the question implies that such normal PA pressure has been observed in patients who have disease severe enough to require ICU care with or without mechanical ventilation.
There is considerable unperfused reserve in the normal resting pulmonary capillary bed. This is demonstrated by pneumonectomy studies which show that loss of a single lung (approximate halving of the capillary bed) characteristically does not cause any increase in PA pressure at rest. There is also more recent demonstrations that even at very modest levels of exercise (doubling of cardiac output) the functional capillary bed in humans approximately doubles, a further demonstration of the large reserve available in the pulmonary circulation.
The implication of pulmonary circulatory reserve in the current context is that when a lung disease is “patchy” or heterogenous, there is the capacity in the remaining normal lung to accommodate the cardiac output without an increase in PA pressure. Based on CT appearances the initial pattern of lung involvement in Covid-19 is patchy and therefore might lead to diversion of blood flow to relatively normal areas of lung without any significant rise in PA pressure.
A second possible mechanism for the preservation of normal PA pressure in the presence of extensive areas of lung disease (hypoxic alveoli) particularly with alveolar “flooding” is that hypoxic pulmonary vasoconstriction is antagonized in Covid-19 associated lung disease. This would keep PA pressure low by maintaining a low pulmonary vascular resistance. Furthermore, it would potentiate the shunt effect on non-ventilated regions (such as areas of alveolar oedema) with a consequent worsening of systemic arterial hypoxaemia. This fits with the profound and often refractory arterial hypxoxaemia observed in Covid-19.
Inhibition of hypoxic pulmonary vasoconstriction by inflammation induced by sepsis has been shown experimentally in several different animal species.
Strategies for inhibition of cycloygenase, lipoygenase and nitric oxide synthase pathways have been investigated as ways to reverse this inhibition of hypoxic pulmonary vasoconstriction. The results have been somewhat variable. Inhibition of inducible nitric oxide synthase is that intervention that has most frequently been reported to restore hypoxic pulmonary vasoconstriction.
Potentially an inhaled vasodilator (prostacyclin or nitric oxide) might help to divert blood flow to better ventilated areas although in other forms of ARDS inhaled NO has not been shown to reduce mortality, although it does improve arterial oxygenation.
In the late stage of Covid-19 lung disease PA pressure might well increase due to the lung disease becoming more extensive (less normal lung to act as a capillary “reserve”), the progression of thrombotic vascular obstruction and the development of pulmonary vascular remodelling with vascular reduction.
Early anticoagulation in some patients patients admitted to ICU might prevent progression of thrombotic disease and in one recently published retrospective study, has been associated with better outcome in patients with the highest concentrations of D-dimers (> 6 times the upper limit of normal) and other evidence of disseminated intravascular coagulation (Tang et al J Thromb Haemost March 2020. PMID: 32220112 DOI: 10.1111/jth.14817)
Further response [posted 24/04]
Regarding the question of changes in pulmonary artery pressure a preprint (as yet unreviewed) article was posted (https://doi.org/10.1101/2020.04.06.20050575) recently reporting on the pulmonary and cardiac findings at post mortem in a small series of four patients who died from severe Covid-19. All four had were treated in ICU and mechanically ventilated.
The lungs were grossly abnormal with multiple well demarcated regions of haemorrhage but no evidence of thromboembolic disease. Microscopic examination showed extensive microvascular thrombosis in the alveolar capillaries and small vessels.
Examination of the hearts (in three cases) showed enlargement with dilatation of the right ventricle. There was no evidence of coronary artery stenosis or thrombosis and macroscopically the cut surface of the myocardium was free of visible lesions in all cases. Microscopic examination demonstrated that there were no areas of confluent necrosis although scatted single myocyte necrosis was seen. There was no evidence of myocarditis.
The right ventricular dilatation reported here in the absence of myocarditis or ischaemic heart disease is compatible with right ventricular failure due a sudden increase in pulmonary artery pressure pressure caused by reduction in the pulmonary vascular bed due to the extensive microvascular thrombosis and (presumed) alveolar hypoxia. The right ventricle does not tolerate acute increases in pressure load and dilates rapidly in response to such an increase.
7: Cardiac troponins [RESPONDED]
Question 7: Cardiac troponins are massively elevated. What does this mean?
Load-independent changes in cardiac contractility over time- can we measure?
Response: Two studies have shown an increased risk of mortality associated with elevated troponin levels (both from Wuhan, China) and one of these studies also showed an association with elevated BNP and CRP. In the first study by Shi and colleagues of 416 patients, those with elevated troponin I had significantly higher mortality (51% vs. 4.5%) and in the second study by Guo et al. of 187 confirmed COVID-19 hospitalized patients, those with elevated troponin T had a mortality of 59.6% vs. 8.9% in those with normal troponin T levels. Although mortality rates were highest in those with cardiovascular disease and elevated troponin levels (a staggering 69.4%), those without underlying cardiovascular disease who had an elevated troponin still had a higher mortality (37.5% died) compared to those with normal troponin levels. It’s becoming clear that myocardial injury and myocarditis can accompany pneumonia and portend a poorer prognosis. Recently, a case report from Italy described a patient without cardiovascular diseased that developed myocarditis from COVID-19, this was her sole presentation (no lung disease). Therefore, COVID-19 has an affinity for the heart as well as the lung and elevated troponin levels portend a higher risk of severe illness and death, even in patients without underlying cardiovascular disease.
8. PPE and CPAP developments [RESPONDED]]
Question 8: What are the latest developments in alternative PPE and CPAP provision in terms of physiological testing?
There are a number of innovative projects in the area of PPE ongoing at the moment, from the 3D printing and laser cutting of face shields to the use of a variety of alternatives (e.g. masks) for protection and the provision of CPAP and ventilation. The Advisory Panel is happy to try and address specific questions on PPE in the area of physiology; there will also be separate items on The Physiological Society website of some of the work being undertaken in this area
Response [13/04/2020]
That a pandemic might cause a shortage of personal protective equipment (PPE), particularly respiratory devices, had been concluded by several studies in the past (Radonovitch et al. 2008; Rengasamy et al. 2010). Studies were conducted to test the respiratory protection offered by textiles available in most households (Davies et al. 2013). The conclusion from these studies is that such homemade masks offer only marginal protection.
Current efforts employing innovative concepts should consider the following:
1) Design and construction of face masks. At the request of physicians, we recently tested the particle removal efficiency (PRE,%) of a variety of face masks. This was conducted by placing a face mask on a head manikin, and initiating a flow of air (4 L.min-1) through the face mask in the direction of inspiration. During the test the head manikin is in a chamber containing an aerosol of nanoparticles of different sizes. The test allows the determination of the removal of different sized particles. This is an important test, as it provides information of the PRE of particles of the same size as a virus (i.e. 70-100 nm). We also measured PRE of the materials. The efficiency of a mask is very much dependent not only on the material, but also the design.
2) Work of breathing. This is normally not an issue with face masks, however respiratory devices that impose a high inspiratory and/or expiratory resistance are unacceptable. The work of breathing can be measured by placing the respiratory device on a head manikin, the mouth of which is connected to a breathing simulator. During simulated breathing, inspiratory and expiratory pressure is measured during in the inspired and expired volume, respectively. Integration of the pressure-volume loop provides the work of breathing.
3) Dead space. Respiratory devices have a specific volume, which contributes to the dead space volume. Inspired and expired gases (oxygen and carbon dioxide) are mixed in the dead space of the mask. Depending on the minute ventilation and tidal volume, we can anticipate an accumulation of carbon dioxide and depletion of oxygen in this dead space. It is therefore important to measure the carbon dioxide and oxygen levels in the mask during normal operation. Industrial standards suggest that the 8-hour weighted average for carbon dioxide should not exceed 0.5%. Standards also suggest that the oxygen should be maintained above 19%.
The modification of snorkeling face masks into respiratory protective devices has received much attention of late. The endeavours of the engineers and innovators that have provided (free) instructions for the refurbishment of such masks is commendable. We have tested such face masks and concluded the following:
1) Work of breathing is satisfactory.
2) Particle removal efficiency is good.
3) CO2 accumulation is unacceptable.
4) O2 depletion is unacceptable.
The source of the accumulation of CO2 and depletion of O2 is the design of the mask. The mask comprises a full face mask, which seals on the face, and an inner oronasal mask, which should also provide a proper seal on the face. During tests, we observed that some masks do not provide a proper seal of the oronasal part of the mask, and thus the dead space volume is increased. It should be noted that a number of fatalities have occurred using these masks (Wu 2019), which have been attributed to CO2 accumulation (Davis 2019).
Modification of gas masks for use by physicians. There are a range of gas masks commercially available. They are used by firefighters, military, etc. They work on the same principal as the recreational snorkeling masks, but have a far superior design. Particularly of the oronasal mask. We have modified these masks for physicians, such that an adapter allows the attachment of medical filters. Our tests have demonstrated that:
1) Work of breathing is satisfactory.
2) Particle removal efficiency is good.
3) CO2 accumulation is satisfactory.
4) O2 depletion is satisfactory.
Modification of gas masks into CPAP masks. We have recently made adapters for the inlet (filter port) and outlet (expiratory port) ports of these masks, so that they could be connected to a CPAP device providing an oxygen-enriched air mixture. By attaching a CPAP valve on the expiratory side of the gas mask, a desired positive pressure can be maintained in the mask. The expired gas is expelled from the mask through a medical filter. These modifications are currently being tested, and should be evaluated within a few days.
Physiological testing. Our experience is that it is absolutely of paramount importance to conduct physiological tests of respiratory devices. This can be conducted with appropriate simulators (i.e. head manikin connected to a breathing simulator). Due to the impending disaster that may befall a healthcare system in a country dealing with the COVID-19 virus, it is understandable that solutions cannot be subjected to lengthy verification and certification procedures, but it would be negligent not to conduct basic tests to ensure that a simple face mask is not creating more problems than it is trying to solve.
In 2006 the National Academy of Sciences published the findings of the Committee on the Development of Reusable face masks for use during an influenza pandemic, reflecting their concern that such a pandemic may require a strategy of reusing (disposable) face masks. In their introduction the quote from the German poet Goethe is very prophetic.
Acknowledgements
I would like to acknowledge the input of colleagues to this work.
Bibliography
Beder A., Büyükkoçak U., Sabuncuoğlu H., Keskil Z.A., Keskil S. (2008). Preliminary report on surgical mask induced deoxygenation during major surgery. Neurocirurgia 19: 121-126.
Center for Disease Control (2020). Carbon dioxide. National Institute for Occupational Safety and Health. www.cdc.gov/niosh/pel88/124-38.html (accessed on April 7, 2020)
Chughtai A.A., Seale H., MacIntyre C.R. (2013). Use of cloth masks in the practice of infection control-evidence and policy gaps. Int. J. Infect. Control 2013 v9:i3. doi: 10.3396/IJIC.v9i3.020.13.
Craig Jr. A.B. (1961). Underwater swimming and loss of consciousness. JAMA 176: 87-90.
Craig Jr. A.B. (1976). Summary of 58 cases of loss of consciousness during underwater swimming and diving. Med. Sci. Sports Exer. 8:171-175. doi: 10.1249/00005768-197600830-00007.
Davies A., Thompson K.-A., Giri K., Kafatos G., Walker J., Bennett A. (2013). Testing the efficacy of homemade masks: Would they protect in an influenza pandemic? Disaster Med Pub Health Preparedness 7: 413-418.
Davis C. (2019). Spike in snorkel-related deaths again highlights potential danger of full-face masks. Hawaii New Now September 17, 2019 (accessed on April 7, 2020).
European Industrial Gas Association, EIGA (2018). Hazards of oxygen deficient atmospheres. Doc. 44/18, Revision of Doc 44/09; EIGA Aisbl: Brussels, Belgium
Florio, J.T. , Morrison J.B. and Butt, W.S. (1979). Breathing pattern and ventilatory response to carbon dioxide in divers. J. Appl. Physiol. 46: l076-l080.
Fothergill, D.M., D. Hedges, and J.B. Morrison, 1991. The effects of CO2 on cognitive and psychomotor performance in the hyperbaric environment. Undersea Biomed. Res., 18: 1-19.
Grasselli G., Pesenti A., Cecconi A. (2020). Critical care utilization for the COVID-19 outbreak in Lombard, Italy. Early experience and forecast during an emergency response. JAMA. Doi:10.1001/jama.2020.4031.
Hesser, C. M., J. Adolfson, and L. Fagraeus. Role of CO2 in compressed-air narcosis. Aerosp. Med. 42: 163-168, 1971.
Kim J.-M., Chung Y.-S., Jo H.-J., Lee N.-J., Kim M.S., Woo S.H., Park S., Kim J.W., Kim H.M, Han M.-G. (2020). Identification of coronavirus isolated from a patient in Korea with COVID-19. Osong Public Health Res Perspect 11:3-7. doi:10.24171/j.phrp.2020.11.1.02
Lim ECH, Seet RCS, Lee K-H, Wilder-Smith EPV, Chuah BYS, Ong BKC (2006). Headaches and N95 face-mask amongst healthcare providers. Acta Neurol Scand 113: 199-202. doi:10.1111/j.1600-0404.2005.00560.x
Livingston E., Berkwits M. (2020). Sourcing personal protective equipment during the COVID-19 pandemic. JAMA (published online March 28, 2020). doi: 10.1001/jama.2020.5317.
Lun V., Sun J., Passias T., Mekjavic I.B. (1993). Effects of prolongued CO2 inhalation on shivering thermogenesis during cold-water immersion. Undersea Hyper Med 20: 215-224.
MacIntyre C.R., Chughtai A.A. (2015). Facemasks for the prevention of infection in healthcare and community settings. BMJ 350: h694. doi: 10.1136/bmj.h694.
MacIntyre C.R., Seale H., Dung T.C., Hien N.T., Nga P.T., Chughtai A.A., Rahman B., Dwyer D.E., Wang Q. (2015). A cluster randomised trial of cloth masks compared with medical masks in healthcare workers. BMJ Open 5: e006577. doi: 10.1136/bmjopen-2014-006577.
Martyny J., Glazer C.S., Newman L.S. (2002). Respiratory protection. N Engl. J. Med. 347: 824-830.
Mekjavic I.B., Amon M., Kolegard R., Kounalakis S.N., Simpson L., Eiken O., Keramidas M.E., Macdonald I.A. (2016). The effect of normobaric hypoxic confinement on metabolism, gut hormones, and body composition. Front. Physiol. 7:202. doi: 10.3389/fphys.2016.00202
Morishima M., Mitsuno T. (2019). Analysis of hygenic face mask patterns for young people. Textile Res. J. 89: 4670-4680.
Morrison, J.B. and S.D. Reimers (1982a). Design principles of underwater breathing apparatus. In: The Physiology and Medicine of Diving. Ed. P.B. Bennett and D.H. Elliott, 3rd Ed., pp.55-98. Bailliere Tindall, London
Morrison, J.B., J.T. Florio, A.G. Thornton and M.K. Todd (1982b). Proposed unmanned test procedures and physiological acceptance criteria. J. Soc. Underwater Tech. 8: 24-28.
Quesnel L.B. (1975). The efficiency of surgical masks of varying design and composition. Br. J. Surg. 62: 936-940.
Radonovich Jr. L.J., Cheng J., Shenal B.V., Hodgson M., Bender B.S. (2009). Respirator tolerance in health care workers. JAMA 301: 36-38.
Rengasamy S., Eimer B., Shaffer R.E. (2010). Simple respiratory protection- evaluation of the filtration performance of cloth masks and common fabric materials against 20 – 200 nm size particles. Ann. Occup. Hyg 54: 789-798. doi: 10.1093/annhyg/meq044.
Rucki A. (2020). Engineers in Italy have transformed scuba diving masks into ventilators – they could now be arriving at UK hospitals to treat coronavirus patients. Manchester Evening News April 1 2020 (accessed April 7, 2020).
Saatci E., Miller D.M., Stell I.M., Lee K.C., Moxham J. (2004). Dynamic dead space in face masks used with noninvasive ventilators: a lung model study. Eur. Respir. J. 23: 129-135.
Sayers JA, Smith REA, Holland RL, Keatinge WL, 1987 Effects of carbon dioxide on mental performance. J. APPL. PHYSIOL., 63: 25-30.
Shaw K.S., Ngan Kee W.D., Tam Y.H., Wong M.K., Lee S.W.Y. (2008). Survey and evaluation of modified oxygen delivery devices used for suspected severe acute respiratory syndrome and other high-risk patients in Hong Kong. Hong Kong Med. J. 14 (Supplement 5): S27-31.
Van der Sande M., Teunis P., Sabel R. (2008). Professional and home-made face masks reduce exposure to respiratory infections among the general population. PloS One 3(7): e2618. doi:10.1371/journal.pone.0002618.
Wu H.-L., Huang J., Zhang C.J.P., He Z., Ming W.-K. (2020). Facemask shortage and the novel coronavirus diesease (COVID-19) outbreak: Reflection on public health measures. EclinicalMedicine. doi:10.1016/eclinm.2020.100329.
Wu N. (2019). Tour operators start ban on full-face snorkel mask. The Honolulu Star. Sept. 24, 2019 (accessed at www.cbsnews.com/news/hawaii-full-face-snorkel-mask-related-deaths/ on April 7, 2020).
9. Vitamin C [RESPONDED]
Question 9: A common question or request when phoning relative of patients who are in ITU is “have we given VITAMIN C” and if please can we try it. Is there any evidence for benefit or harm in giving it?
Response [13/04/2020]:
Summary
There is no robust evidence to support the use of IV Vitamin C in the management of sepsis or COVID-19 related pneumonia. IV Vitamin C is relatively safe at doses lower than 7.5 grams/day, but not without risk.
Background
Vitamin C deficiency has been identified in critically ill sepsis patients, but it’s clinical utility is not well established (Wilson 2009). A small series of 37 patients with severe (>30% TBSA) thermal burns showed a reduction in the amount of crystalloid fluid resuscitation and severity of respiratory dysfunction as defined by a reduction in ventilator days. (Tanaka 2000). A series of 595 patients, 91% of whom were victims of trauma, were randomized to receive IV Vitamins C and E. There was a trend toward lower rate of respiratory complications, but not statistically significant. Secondary endpoints of decreased ICU length of stay and organ failure were achieved. (Nathens 2002)
A 2014 Phase I trial of 24 ICU patients with severe sepsis were randomized to either placebo, high, or low dose IV vitamin C and showed safety and tolerability. (Fowler 2014). Vitamin C is a required co-factor in catecholamine synthesis and showed reduced mortality and requirement for vasopressors in a study of 127 ICU patients (Carr 2015).
Discussion
Numerous small studies support the relative safety of IV vitamin C. There is a single case report of oxalate crystal nephropathy in high dos Vitamin C (Buehner 2016). There is a theoretical pro-oxidative effect of Vitamin C, but this wasn’t demonstrated in a single study of 6 healthy adult males. In patients with high ferritin, free iron is released to form ferritin under hypoxic conditions and may contribute to the pro-oxidant effect of vitamin C. Vitamin C also increases iron absorption in persons with iron storage disorders (Lane 2014). Mechanistic pathways are proposed in the treatment of COVID-19 patients, but have not been studied in controlled populations (Erol 2020).
On March 27, 2020, the Australian Government Department of Health, Therapeutic Goods Administration issued guidance indicating that there was no evidence to support the role of Vitamin C in the management of COVID-19. www.tga.gov.au/alert/no-evidence-support-intravenous-high-dose-vitamin-c-management-covid-19 (accessed Apr 12, 2020)
There is currently a clinical trial underway to further explore Vitamin C in patients with COVID-19. ( Carr, A.C. A new clinical trial to test high-dose vitamin C in patients with COVID-19. Crit Care 24, 133 (2020). doi.org/10.1186/s13054-020-02851-4)
References
drive.google.com/file/d/1ysihwB2JXQx_W_IHD3YN5ejPKRg79uVN/view?usp=sharing
10. Vitamin D [RESPONDED]
Question 11: If the patients with COVID19 who have been seriously ill, requiring ventilation etc. have vitD deficiency or is it unknown? Are these patients tested for Vit D?
Response
There is a plausible hypothesis that vitamin D deficiency, whilst probably not impacting on risk of infection from SARS-CoV-2 coronavirus, might greatly increase risk for COVID-19 severity and mortality.
Evidence that vitamin D deficiency could contribute to risk of cytokine storm and hence risk of ARDS/death from COVID19 is as follows:
1. There is currently a markedly reduced mortality from COVID19, expressed per million population, in countries that are south of 35 degrees latitude North – the point above which many people (probably around 30% in UK at present) will currently be vitamin D deficient because of insufficient ultraviolet (UVB) exposure. This association between mortality and latitude is highly significant P<0.0001:
Rhodes JM, Subramanian S, Laird E, Kenny RA. Editorial: Low population mortality from COVID-19 in countries south of latitude 35 degrees North – supports vitamin D as a factor determining severity. Aliment Pharmacol Therap 2020 DOI: 10.1111/apt.15752
2. People with dark skin are much less readily able to synthesize vitamin D with sunlight exposure and there is a very strong association between ethnicity and vitamin D deficiency, eg 8-fold increased risk of vitamin D deficiency (17.5%, 95% CI 15.2-20.0) in non-hispanic black) in a 16,180 US cohort compared with 2.1%, 95% CI1.5-2.7 in non-hispanic whites. This parallels the much greater mortality being reported from COVID-19 amongst dark-skinned ethnic minorities (10-fold increase amongst Swedish Somalis being reported in Swedish media).
Herrick KA, Storandt RJ, Afful J, et al. Vitamin D status in the United States, 2011-2014. Am J Clin Nutr 2019;110:150-157.
3. Vitamin D deficiency is also associated with diabetes, hypertension, obesity, living in institutions – all of which are also risk factors for COVID-19 mortality.
Kunutsor SK, Apekey TA, Steur M. Vitamin D and risk of future hypertension: meta-analysis of 283,537 participants. Eur J Epidemiol 2013;28:205-21.
Mauss D, Jarczok MN, Hoffmann K, Thomas GN, Joachim E. Fischer JE. Association of Vitamin D Levels with Type 2 Diabetes in Older Working Adults. Internat J Med Sci 2015; 12:362-368.
Yao Y, Zhu L, He L, et al. A meta-analysis of the relationship between vitamin D deficiency and obesity. Int J Clin Exp Med 2015;8:14977-84.
Roth DE, Abrams SA, Aloia J, Bergeron G, Bourassa MW, Brown KH, et al. Global prevalence and disease burden of vitamin D deficiency: a roadmap for action in low- and middle-income countries. Ann N Y Acad Sci 2018;1430:44-79.
4. There is considerable experimental evidence showing that vitamin D modifies and reduces the cytokine response to pathogens. This has been shown with respiratory epithelial cell lines, macrophages and in animal models. There is evidence that this effect is mediated to a substantial extent by upregulation of ACE2 and in particular an increase in the ratio of ACE2:ACE – this results in more rapid hydrolysis of angiotensin II which is thought to be a major stimulant of cytokine storm/ARDS.
Greiller CL, Martineau AR. Modulation of the immune response to respiratory viruses by vitamin D. Nutrients 2015;7:4240-70.
Arboleda JF, Fernandez GJ, Urcuqui-Inchima S. Vitamin D-mediated attenuation of miR-155 in human macrophages infected with dengue virus: Implications for the cytokine response. Infect Genet Evol. 2019;69:12-21.
Puerta-Guardo H, Medina F, De la Cruz Hernández SI, Rosales VH, Ludert JE, del Angel RM. The 1α,25-dihydroxy-vitamin D3 reduces dengue virus infection in human myelomonocyte (U937) and hepatic (Huh-7) cell lines and cytokine production in the infected monocytes. Antiviral Res. 2012;94:57-61. Erratum in: Antiviral Res. 2012 Jun;94(3):297. Medina, Fernando [added].
Villamor E, Villar LA, Lozano A, Herrera VM, Herrán OF. Vitamin D serostatus and dengue fever progression to dengue hemorrhagic fever/dengue shock syndrome. Epidemiol Infect. 2017;145:2961-2970.
Zhang Y, Leung DY, Richers BN, et al. Vitamin D inhibits monocyte/macrophage proinflammatory cytokine production by targeting MAPK phosphatase-1. J Immunol 2012;188:2127-35.
Kong J, Zhu X, Shi Y, et al. VDR attenuates acute lung injury by blocking Ang-2-Tie-2 pathway and renin-angiotensin system. Mol Endocrinol. 2013;27:2116-25.
Tsujino I, Ushikoshi-Nakayama R, Yamazaki T, Matsumoto N, Saito I. Pulmonary activation of vitamin D3 and preventive effect against interstitial pneumonia. J Clin Biochem Nutr. 2019;65:245-251. Xu J, Yang J, Chen J, Luo Q, Zhang Q, Zhang H. Vitamin D alleviates lipopolysaccharide induced acute lung injury via regulation of the renin angiotensin system. Mol Med Rep. 2017 Nov;16(5):7432-7438.
Yuan W, Pan W, Kong J, et al. 1,25-dihydroxyvitamin D3 suppresses renin gene transcription by blocking the activity of the cyclic AMP response element in the renin gene promoter. J Biol Chem. 2007;282(41):29821–29830.
5. Human evidence of increased lung damage with viral infection in vitamin D deficiency is mainly based on studies of Respiratory Syncytial virus (RSV) infection in infants where vitamin D deficiency, and separately, vitamin D receptor polymorphisms, have been shown to be associated with increased risk of requiring intensive care.
Vo P, Koppel C, Espinola JA, et al. Vitamin D Status at the time of hospitalization for bronchiolitis and its association with disease severity. J Pediatr 2018;203:416-422.
McNally JD, Sampson M, Matheson LA, Hutton B, Little J. Vitamin D receptor (VDR) polymorphisms and severe RSV bronchiolitis: a systematic review and meta-analysis. Pediatr Pulmonol 2014;49:790-9.
6. Studies of vitamin D levels correlated with outcomes and RCTs of vitamin D supplementation are currently ongoing in COVID-19. The only published data to date is a non-peer reviewed pre-print that reports a very strong association between vitamin D deficiency and severe COVID -19 illness in hospitalised patients in the Phillipines.
Alipio, M. Vitamin D supplementation could possibly improve clinical outcomes of patients infected with coronavirus-2019 (COVID-2019) (April 8, 2020). Available at SSRN: ssrn.com/abstract=3571484
or dx.doi.org/10.2139/ssrn.3571484
This and other similar studies of vitamin D levels in people who are already ill will have to be interpreted with caution since there is clear evidence that serum vitamin D levels fall during the acute phase response to illness:
Nonnecke BJ, McGill JL, Ridpath JF, Sacco RE, Lippolis JD, Reinhardt TA. Acute phase response elicited by experimental bovine diarrhea virus (BVDV) infection is associated with decreased vitamin D and E status of vitamin-replete preruminant calves. J Dairy Sci. 2014;97(9):5566–5579.
Silva MC, Furlanetto TW. Does serum 25-hydroxyvitamin D decrease during acute-phase response? A systematic review. Nutr Res. 2015;35(2):91–96.
11. Oxygenation
Question 12: Regarding tea and oxygenation did they see a reduction in the requirement for proning or renal replacement?
12. Hydroxychloroquine [RESPONDED]
Question 12: Had there been a retrospective analysis of the Rheumatoid arthritis patient on Hydroxychloroquine Vs general patient infected with covid?
Response: None that we are aware of, although these data would be important to view. Randomised clinical trials are underway to test the efficacy of HCQ.
13. Proning [RESPONDED]
Question 13: In early COVID-19 respiratory failure, lung compliance and volume are essentially normal and the hypoxaemia appears to be due to V/Q mismatch as a result of high pulmonary blood flow through relatively normally ventilated lungs. In the light of this do we understand why proning in these early patients improves oxygenation when the normally accepted mechanisms in ARDS (improved and more homogenous V:Q and changes in lung shape) would seem to have less effect? Proning appears to have worked in these patients both on ventilators and breathing spontaneously.
Response:
The questioner includes the following statement: “the hypoxaemia appears to be due to V/Q mismatch as a result of high pulmonary blood flow through relatively normally ventilated lungs”. It is not likely that a high flow through relatively normally ventilated lings can be an accurate description. A timely paper by Tian et al (2020) shows the lung pathology in two cases of Covid-19 accidentally made available by lung cancer surgery in patients retrospectively found to have Covid-19 at the time of surgery. The early disease shows much alveolar damage, including oedema, with vascular congestion (https://www.jto.org/article/S1556-0864(20)30132-5/fulltext). The likely pattern of V/Q mismatch at this early stage is perhaps one of areas of unventilated alveoli with a disproportionately large blood flow. In so far as it may be possible to alter the fairly marked top-to-bottom distribution of pulmonary blood flow by change of posture, proning would have the potential to match blood flow better to areas of lung less severely affected and therefore better ventilated. Proning might, of course, also redistribute pulmonary oedema. The pathology seen at a later stage of the disease includes hyaline membranes, as noted by Xu et al (2020) (https://www.thelancet.com/pdfs/journals/lanres/PIIS2213-2600(20)30076-X.pdf). This may be more typical of ARDS seen from other causes and may respond differently to proning, etc.
14. Sodium levels [RESPONDED]
We are seeing profound hypernatremia in our Covid icu patients. This may be due to the profound insensible losses.
The provision of free water is crucial
What are the thoughts about target levels of sodium ; especially when weighed against the need for “dry lungs” in these critically ill patients.
Response:
We were seeing these very high sodiums initially and I think they did coincide with severe dehydration from hyperpyrexia. We’ve eased up on drying out patients now as their ventilation seems to tolerate euvolaemia and they have sodiums at the upper end of normal. The AKI patients that we have started on peritoneal dialysis often absorb some of the dialysis fluid if they are very dry and a few have subsequently recovered renal function and have been extubated.
15. Asthma sufferers [RESPONDED]
Question 15: is there any evidence that inhaled steroids taken by asthma sufferers are actually protective against the more serious pulmornay effects of Covid19?
Response:
Two preprints (not yet refereed) report an inhibitory action of the inhaled steroid ciclesonide on SARS-CoV2 replication in cell culture. In one of these reports, this action action is specifically against SARS-CoV2; ciclesonide did not inhibit respiratory syncytial virus or influenza virus replication. www.biorxiv.org/content/10.1101/2020.03.11.987016v1.abstract and
www.biorxiv.org/content/10.1101/2020.03.20.999730v1.abstract
The issue of the clinical use of inhaled steroids to treat CoVID-19 in patients has been recently discussed by Halpin and colleagues in a rapid systematic review (https://erj.ersjournals.com/content/early/2020/04/20/13993003.01009-2020#ref-29).
The authors conclude that at present there is no evidence for a therapeutic benefit of instituting inhaled steroid therapy in patients with CoVID-19.
Two trial of the use of inhaled steroid (i.e. instituting inhaled steroid therapy in patients with CoVID-19 who are not already taking these agents for asthma or COPD) are currently under way.
clinicaltrials.gov/ct2/show/NCT04330586 and
clinicaltrials.gov/ct2/show/NCT04331054
However, patients with asthma or COPD who are already on inhaled steroid therapy should continue to take their medications.
The authors note that patients with asthma and COPD appear to be under represented among those suffering from CoVID-19, although the reasons for this are at present unknown.
16. Hypoxaemia [RESPONDED]
Question 16: The mechanism of hypoxaemia in the early phenotype (Gattinoni “L”) of COVID-19 appears to be V/Q mismatch due to unregulated pulmonary vasodilatation but some people are anecdotally reporting successful use of inhaled NO or prostacyclin in these patients. Do we have any idea of the mechanism of action of an inhaled pulmonary vasodilator to improve oxygenation in patients who already have a dilated pulmonary circulation?
Response [19/04/2020]
Inhaled nitric oxide therapy may go beyond lung vascular effects. One possibility is that nitric oxide inhalation will reduce oedema and inflammation. There are papers on this and here is one example:
Inhaled nitric oxide caused anti-inflammatory effects as observed through reduced oedema, mieloperoxidase activity and neutrophil infiltration (Coelho et al. 2015).
In a rabbit model of ARDS; Treatment with intratracheal SNAP alleviated lung injury and edema and improved lung functions (including ventilation/blood gases) in a saline-lavaged model of ARDS
It’s recognised that high levels of NO from endogenous activation of iNOS may be cytotoxic so attention to dose should be considered.
Coelho CF et al. 2015. Histol Histopathol. 30:117-24. doi: 10.14670/HH-30.117.
Kosutova P et al. (2019). Physiol. Res. 68(Suppl 3):S265-S273.
Also,nitric oxide has an anti-platelet function; this may help with better ventilation and V/Q matching.
Liu et al. (2015). Thromb Res. 136:319-27. doi: 10.1016/j.thromres.2015.05.016.
17. Harvesting serum from cured COVID-19 sufferers [RESPONDED]
Question 17: Why is the Government’ planning to accelerate Vaccine studies against COVID-19 which will take ages to eliminate the 800 deaths a day at present while doing next to nothing about harvesting serum from cured COVID-19 sufferers. Anyone aware about the history of control of Diphtheria will know, until you have heard immunity, Vaccines will be of no use in reducing mortality from a disease that is likely to kill in 7-14 days after admission to ICU. Use of cured serum therapy, in contrast, has been known since the time of 1917/18 flu epidemic to cure a large number of dying patients and in 2015 Nottingham scientist published a systematic review of 1320 cases in studies of cured serum therapy in respiratory failure from a variety of Viral diseases, including Coronaviruses and demonstrated a 75% reduction of death.
Response:
Duan et al treated 10 severe COVID patients with a single dose of convalescent plasma and noted good recovery with no adverse effects. (attached)
Shen et al published a study of 5 critical patients with recovery after convalescent plasma. (attached)
Good opinion article in the Journal of Clinical Investigation on the history and case for it’s use. (attached)
The United States Food and Drug Administration (Equivalent to MHRA) allows for emergency experimental treatment and there are ongoing trials. www.fda.gov/vaccines-blood-biologics/investigational-new-drug-ind-or-device-exemption-ide-process-cber/recommendations-investigational-covid-19-convalescent-plasma
It looks like the MHRA and NHS are also conducting trials. pharmafield.co.uk/pharma_news/coronavirus-convalescent-plasma-wanted-for-clinical-trial/
18. Arginase inhibitors [RESPONDED]
Question 18: Is there a role for Arginase inhibitors to restore hypoxic pulmonary vasoconstriction in COVID-19?
Response
There are two published studies that report the effects of arginase manipulation on acute hypoxic pulmonary vasoconstriction. Both studies were conduced in mice but suggest very different actions of arginase.
In a very thorough study using genetically manipulated mice, Cowburn et al (PNAS 2016) found that deletion of endothelial arginase1 protected against the development of hypoxic pulmonary hypertension and also showed that deletion of endothelial HIF2alpha (which acts to promote arginase1 expression) caused a reduction of acute hypoxic vasoconstriction. Many previous publication report that inhibition of arginase (1 and 2) attenuate the development of hypoxic pulmonary hypertension, although those did not report the effect of arginase inhibition on acute hypoxic pulmonary vasoconstriction. However, it has been repeatedly demonstrated that interventions that increase nitric oxide (a important effect of arginase inhibition) reduce hypoxic vasoconstriction in the lung.
Petersen et al more recently reported that arginase1 is increased in mice treated with endotoxin and that those endotoxaemic mice had impaired acute hypoxic pulmonary vasoconstriction. They found that arginase inhibition restored acute hypoxic vasoconstriction in the lungs of the endotoxaemic mice. As they acknowledged in their discussion, this finding was “counterintuitive” given the prior literature and speculated that the effect was not directly the result of arginase inhibition and any resultant increase in nitric oxide, but may have resulted from alternative actions (anti-oxidant effects or actions on ion channels).
There are multiple previous papers showing that reduction in arginase action increases nitric oxide (through increased substrate availability for NOS). Furthermore, it is very well demonstrated that nitric oxide attenuates acute hypoxic pulmonary vasoconstriction. Taken with the work of Cowburn et al (PNAS 2016), the balance of evidence suggests that arginase inhibition would not restore acute hypoxic pulmonary vasoconstriction in patients with COVID-19, although the work of Petersen et al remains to be explained. Nonetheless, the available evidence does not at present support the use of arginase inhibitors to improve hypoxic pulmonary vasoconstriction in humans.
19. Thromboxane [RESPONDED]
Question 19: Thromboxane is released in response to hypoxia and acute phase reactants, is there a role for inhibitors of thromboxane synthesis or thromboxane receptor antagonists in COVID-19?
Response: 2/05/20
I couldn’t find any published or grey literature on this topic or mechanism. Aspirin is probably the most ubiquitous TXA-2 inhibitor. There is a clinical trial underway to assess aspirin in COVID-19 with primary outcome of time to clinical recovery and time of SARS-CoV-2 overcasting. clinicaltrials.gov/ct2/show/NCT04365309
There seems to be some discussion about the importance of continuing usual prophylactic cardiovascular doses of aspirin in special populations, namely those with pregnancy or cardiovascular risk, but it is anecdotal
https://obgyn.onlinelibrary.wiley.com/doi/epdf/10.1002/uog.22049
20. Interventions that may enhance ventilation/perfusion
Question 20: Physiological and pharmacological interventions that may enhance ventilation/perfusion match and reduce intra-pulmonary shunt:
Mild hyperthermia L-lactic acid infusion TASK-1 channel blocker infusion Desferrioxamine infusion Anandamide infusion Asymmetric dimethylarginine (AMDA) infusion Arginase inhibitor infusion N-Hydroxy-nor-L-arginine (nor-NOHA) infusion Angiotensin II infusion
Beta-2 agonist infusion
Avoid vasodilators (CCB)
Position CoViD lobules upper.
Could some of these measured be trialled in COVID-19 patients? Results should be apparent within minutes measured with pulse oximetry.
21. Gastrointestinal symptoms [RESPONDED]
Question 21: Covid may cause Gastrointestinal symptoms and virus has been detected in stool by PCR but is there infectious risk from stool through aerosol during lower GI endoscopy?
Response: The American Gastroenterological Association recently strongly recommended the use of N95 rather than surgical masks for both upper and lower endoscopy, although the quality of evidence is as yet low for the latter recommendation. They also suggest that all patients who present for endoscopy should be assumed to be possibly infected with coronavirus, and thus precautions should be universally applied until testing is more widespread. GI symptoms are observed in a minority of patients with COVID-19 and viral RNA was detected in stool samples from every member of a hospitalized cohort in a paper published in Gastroenterology, even after clearance of the virus from the respiratory tract. On the other hand, it is not yet clear whether transmissible virus typically persists in faeces
22. Children and COVID-19
Question 22: Why are children less sick with respect to covid 19? Does their development physiology of the lung make them less recruit alvelaor macrophages to inflammation , pulmonary vasoplegia less likely ?
23. Re-infection [RESPONDED]
Question 23: Are there chances of re-infection with COVID-19 once the patient recovers from it? 2)Can a person with COVID-19 infection still transmit the infection to others after clinical recovery from it? What is the time recommended for retesting for the virus after the onset of symptoms/diagnosis of COVID-19 in a patient who becomes symptoms free? 4)Is there confirmed role of Hydroxychloroquine and Azithromycin In prophylaxis and treatment of COVID-19?
Response
Are there chances of re-infection with COVID-19 once the patient recovers from it?
Yes. We do not know how long immunity lasts. For the common cold, one can even catch the disease >1 time in one season. The flu virus mutates, meaning that one can be susceptibe one season if ill/ vaccinated the year before. We don’t yet know cf CV19.
Can a person with COVID-19 infection still transmit the infection to others after clinical recovery from it?
Yes. Some people can keep shedding virus for some time. However, most appear to have very low viral load within 14 days of becoming symptomatic.
Is there confirmed role of Hydroxychloroquine and Azithromycin In prophylaxis and treatment of COVID-19?
No. And no evidence yet cf efficacy in treating. Trials are ongoing for the latter. But it would not be advisable for population-wide prophylactic use of an antibiotic and an agent with a risk of significant side effects (long QT and low platelets, for choloroquine).
24. Citrodiol [RESPONDED]
Question 24: Is there evidence for use of Citrodiol as part of infection preventive strategy?
Response:
We do not have evidence of mosquito vector spread. Citrodiol is converted fro p-menthane-3,8-diol and its topical use would be unlikely to be effective systemically. It dioes appear to have antiviral properties, but dose-ranging is not clear. Nor is duration of action when applied to skin.
25. Guidance for conducting experimental work [RESPONDED]
Question 25: To assist with planning for future research with human participants once Government restrictions are lifted, what guidance / criteria should researchers be following when conducting experimental work:
1. In a laboratory setting
1.1. With ‘healthy’ populations
1.2. With clinical populations
2. In a field-based setting
2.1. With ‘healthy’ populations
2.2. With clinical populations
Response: Please visit here for guidelines we have provided. The Royal Society of Medicine also have a webinar related to this topic. Official UK Government advice ‘Working safely during COVID-19 in labs and research facilities’ was released on 11 May and should be reviewed by people who work in or run indoor labs and research facilities and similar environments.
Please visit here for a document produced as a result of a webinar from 27 May 2020 with over 650 physiologists from over 30 countries about the steps laboratories could take to minimise the risk of transmission of COVID-19 in trials involving humans to both staff and participants.
26. Pathogenetic mechanism of action of SARS-CoV-2 [RESPONDED]
Question 26: We have come upon a very interesting publication (link attached further below) and would like to know your opinion on the proposed pathogenetic mechanism of action of SARS-CoV-2 and whether there are really some evidence-based data that the coronavirus binds the porphyrin and decouples the iron from the heme of the erythrocytes and in this regard do you approve the treatment with chloroquine, azithromycin and zinc (if you do would you please specify at what stage of the clinical evolution). Here is the link to the publication: chemrxiv.org/articles/COVID-19_Disease_ORF8_and_Surface_Glycoprotein_Inhibit_Heme_Metabolism_by_Binding_to_Porphyrin/11938173 I am asking these questions not just on my behalf, but also on behalf of some shy specialists in emergency medicine and resuscitation from my country. Thank you very much in advance for your answer.
Response:
I raised this same question in this blog some weeks ago (11th April Thread #3) when Lui and Li’s paper first surfaced. This was an entirely theoretical in silico modelling of protein-protein interactions. It was not based on any evidence. Since then, this paper has been roundly debunked on the basis their modelling was flawed. See –
Read R (2020): Flawed methods in “Attacks the 1-beta chain of hemoglobin and captures the porphyrin to inhibit human heme metabolism”. ChemRxiv. Preprint. doi.org/10.26434/chemrxiv.12120912
The virus itself is too large to infiltrate the porphyrin ring but the basic idea of this paper was that it was not the virus per se but viral Open Reading Frame proteins transcribed that disrupted the iron binding site of haemoglobin.
Since raising this, we now have experimental data (under review) showing that Hb O2 affinity is unchanged. This does not negate a generalised loss of Hb and anaemic that seems quite common.
So, short answer is No! The theoretical basis of this is flawed and the experimental evidence does not support it at least in terms of Hb O2 affinity.
As a non haematologist, questions I have for haematologists would include –
1. Is the anaemia just an indication of a sick patient or is it more specific? (ie is there ANYTHING in Lui and Li’s paper – however flawed it is?)
2. Is the elevated ferritin seen in COVID patients an indication of generalised organ (liver) failure or is it more specific? (ie is there ANYTHING in Lui and Li’s paper – however flawed it is?)
3. If haem was precipitated out, would this appear as an increase in circulating ferritin (apologies – showing my ignorance of iron metabolism here)?
27. IR fever diagnosis
Question 27: What is the sensitivity and specificity of IR fever diagnosis. Is it a path to pursue, or waste of money?
28. Pre existing arterial disease and arterial dissections
Question 28: Are patients with pre existing arterial disease and arterial dissections at greater risk of vascular complications if they are exposed to Coronavirus? Should they be shielding?
29. COVID-19 transmission
Question 29: There has been a lot of discussion about the risk of transmission with “aerosol-generating procedures”. Is there robust evidence that COVID-19 is transmitted by aerosols/AGPs and not just by droplets?
Research updates
- *NEW* Mechanistic insights into ventricular arrhythmogenesis of hydroxychloroquine and azithromycin for the treatment of COVID-19 – Posted 23/05/20
- Effects of ARBs And ACEIs On Virus Infection, Inflammatory Status And Clinical Outcomes In COVID-19 Patients With Hypertension: A Single Center Retrospective Study
Hypertension
Guang Yang1,2# M.D., Zihu Tan1,2# M.D., Ling Zhou4 # M.D., Min Yang5# Ph.D., Lang Peng1,2# M.M.,
Jinjin Liu1,2 M.M., Jingling Cai1,2 M.M., Ru Yang6 Ph.D., Junyan Han7 Ph.D., Yafei Huang3* Ph.D.,
Shaobin He1,2* M.M. (posted 9/5/20)
- ACE2, COVID-19, and ACE Inhibitor and ARB Use during the Pandemic: The Pediatric Perspective – Andrew M. South, Tammy M. Brady and Joseph T. Flynn (posted 7/5/20)
- Surfing the Waves of the COVID-19 Pandemic As A Cardiovascular Clinician – Payal Kohli and Salim S. Virani (posted 6/5/20)
- Differentiating COVID-19 Pneumonia from Acute Respiratory Distress Syndrome (ARDS) and High Altitude Pulmonary Edema (HAPE): Therapeutic Implications – Stephen L. Archer, Willard W. Sharp and E. Kenneth Weir (posted 6/5/20)
- Effects Of ARBs And ACEIs On Virus Infection, Inflammatory Status And Clinical Outcomes In COVID-19 Patients With Hypertension: A Single Center Retrospective Study – Hypertension (posted 2/05/2020)
- Shiftworking keeps locked-down lab on track – Nature (posted 30/04/2020)
- Strokes Reported Among Some Middle-Aged COVID-19 Patients – Kerry Grens (posted 28/04/2020)
- Nearly All NYC-Area COVID-19 Hospitalizations Had Comorbidities – Lisa Winter (posted 27/04/2020)
Previous research updates
- Blood-pressure drugs are in the crosshairs of COVID-19 research – Deborah J. Nelson (posted 24/04/2020)
- DNA Could Hold Clues to Varying Severity of COVID-19 – Marla Broadfoot (posted 20/04/2020)
- ACEing COVID-19: A Role For Angiotensin Axis Inhibition in SARS-CoV-2 infection? – Ravi Shah , Venkatesh L. Murthy, and Milka Koupenova (Posted 18/04/2020)
- Association of Inpatient Use of Angiotensin Converting Enzyme Inhibitors and Angiotensin II Receptor Blockers with Mortality Among Patients With Hypertension Hospitalized With COVID-19 (Originally published17 Apr 2020) Are Mesenchymal Stem Cells a Promising Treatment for COVID-19? – Ruth Williams (posted 16/04/2020)
- Scientists Scan for Weaknesses in the SARS-CoV-2 Spike Protein – Chris Baraniuk (posted 16/04/2020)
- This Royal Society of Medicine’s webinar series is dedicated to giving healthcare workers on the frontlines, regular and easy-to-access updates from healthcare leaders on COVID-19.
- First Saliva Test for COVID-19 Approved for Emergency Use by FDA – Lisa Winter (posted 15/04/2020)
- The Impact of Camostat Mesilate on COVID-19 Infection (CamoCO-19) – Camostat Mesilate is a serine protease inhibitor that blocks TMPRSS and reduced SARs-Cov-2 binding/entry via ACE2 in vitro and in mice at clinically relevant concentrations. It was approved for clinical use in Japan (for treatment of ulcerative colitis). This clinical trial for SARs-Cov-2 started recruiting in March. (Posted 14/04/20)
- Scientists Scan for Weaknesses in the SARS-CoV-2 Spike Protein – Chris Baraniuk (posted 10/04/2020)
- Are Mesenchymal Stem Cells a Promising Treatment for COVID-19?– Ruth Williams (posted 10/04/2020)
- Blood Pressure Meds Point the Way to Possible COVID-19 reatment – Ashley Yeager (posted 10/04/2020)
- Considerations for Drug Interactions on QTc in Exploratory COVID-19 (Coronavirus Disease 2019) Treatment – Russo et al. (09/04/2020).
[FORUM COMMENT] As the authors point out, Chlorolquine, Hydroxychloroquine, and Lopinavir/ritonavir and azithromycin can all prolong QTc and have an risk of Torsade de Pointes. The risk is especially increased with QTc of 500 msec or more. in general, as this article also points out, a 12 lead ECG should be performed before starting a QT prolonging medication. A different, study, just in JACC EP, shows that one lead ECG is often NOT enough. http://electrophysiology.onlinejacc.org/content/early/2020/04/06/j.jacep.2020.04.001In addition, medications at baseline should be assessed, consideration given to stopping any other QT prolonging medications, baseline electrolytes, including K, M, Ca should be obtained and if abnormal, normalized, prior to starting.Of note, given controversy regarding efficacy of these drugs, careful thought should be given as to whether to start these medications. Randomized data relies on two studies, a small randomized trial of 30 patients total that did not show benefit, and another randomized trial of 62 patients, 31 in each arm, mean age 44+/-15 years, 53% female, that showed only shortened recovery time (shortened fever time by 2 days, and at day 6, there was greater “remission of pneumonia” on CT imaging (80% HCQ vs. 55% in the control group); 4 patients in the control group progressed to severe illness while 0 in the HCQ group did, but this different did not reach statistical significance. - *NEW* Angiotensin Converting Enzyme 2: SARS-CoV-2 Receptor and Regulator of the Renin-Angiotensin System – Oudit et al. (09/04/2020)
- COVID-19 and the Heart – Akhmerov A and Marbán E (08/04/2020).This is a timely general review on Covid and the heart, which highlights more questions than therapeutic answers. The role of many of the potential therapies discussed, whether hydroxychloroquinone or cardiosphere-derived cells (CDCs) remain controversial. The benefit of CDCs for their intended therapeutic role, including i.e. MI and heart failure, has shown mixed results. These lessons points to the fact that emerging therapies should be tested by rigorous randomized trials.
- UK Parliament POST: COVID-19
The Parliamentary Office of Science and Technology (POST) has produced a series of ‘rapid response’ articles on topics including potential vaccines for COVID-19. (08/04/2020) - Scientific Advisory Group for Emergencies (SAGE): Coronavirus (COVID-19) response
The Scientific Advisory Group for Emergencies (SAGE) provides scientific and technical advice to support government decision makers during emergencies. (08/04/2020) - COVID-19: 100 Questions of Peter Piot
Director of the London School of Hygiene and Tropical Medicine, Peter Piot, answers 100 questions on various topics and issues around the COVID-19 pandemic. (08/04/2020)
