
Physiology News Magazine
New actions for familiar vasoactive signals
Recent studies reveal previously unrecognised roles for vasoactive signals in the retinal microvasculature. Donald Puro discusses the physiological and pathobiological implications
Features
New actions for familiar vasoactive signals
Recent studies reveal previously unrecognised roles for vasoactive signals in the retinal microvasculature. Donald Puro discusses the physiological and pathobiological implications
Features
Donald G Puro
Departments of Ophthalmology & Visual Sciences and Molecular & Integrative Physiology, University of Michigan, Ann Arbor, MI, USA
https://doi.org/10.36866/pn.59.33

A traditional notion of vascular physiology is that pericyte-containing microvessels, which are present in all tissues except the liver, play only a passive role in regulating blood flow. However, contrary to this idea it is now becoming clear that, by controlling the contractility of abluminally located pericytes, vasoactive signals can cause microvascular lumens to constrict or to dilate (Schonfleder et al. 1998; Kawamura et al. 2004). These observations point to a new paradigm in which local perfusion is regulated, at least in part, at the capillary level.
In the quest to understand how local perfusion is regulated, a number of investigators have focused on the retinal microvasculature, which is particularly well adapted for the local control of capillary function. One adaptation that facilitates local control is the retina’s lack of autonomic innervation, which in other vascular beds allows extrinsic CNS oversight. In addition, the blood-retinal barrier created by tight junctions between vascular endothelial cells markedly limits the ability of circulating vasoactive molecules to affect perivascular contractile cells. Suggestive of the importance of pericytes in regulating local perfusion within the retina, the density of these cells is higher in this tissue than at any other site in the body.
An experimental advantage of studying the retinal microvasculature is the ability to isolate, from the adult rodent retina, viable complexes of pericytecontaining microvessels (Sakagami et al. 1999). This preparation allows investigators to monitor pericyte currents via patch-pipettes, measure pericyte calcium levels with fura-2, and visualize changes in pericyte contractility and lumen diameter by time-lapse photography. Using this new experimental approach, our laboratory has detected previously unrecognized actions of well known vasoactive signals.
Regulation of cell-to-cell communication
Experiments using freshly isolated retinal microvessels reveal that several vasoactive signals not only affect the contractility of individual pericytes, but also regulate the spatial and temporal dynamics of the vasomotor response (Kawamura et al. 2002, 2003, 2004). Angiotensin II, ATP and endothelin-1 potently inhibit cell-to-cell communication within microvascular complexes, in addition to evoking the release of stored calcium, activating non-specific cation channels and causing pericyte contraction (Fig. 1). The regulation of gap junction pathways is a newly appreciated mechanism by which extracellular molecules regulate microvascular function.

Almost certainly an inhibition of cellto-cell transmission would profoundly influence how a microvascular complex responds to a vasoactive signal. For example, a localized exposure to angiotensin, ATP or endothelin would initially evoke depolarization throughout a network of microvessels as voltage changes induced by the opening of ion channels spreads electrotonically from cell to cell. However, when gap junctions close, intercellular transmission would cease. As a consequence, it is predicted that blood flow would be dynamically affected. Initially perfusion of a relatively large area of the microvasculature would be decreased, but subsequently the inhibition of blood flow would be delimited to the capillary segment directly exposed to one of these extracellular signals. Perhaps the transient inhibition of perfusion in upstream vessels allows for a rapid and effective decrease in capillary blood flow without resulting in a sustained and potentially harmful hypoperfusion of widespread areas within the retina.
Purinergic vasotoxicity
Recent studies indicate that extracellular ATP not only serves as a signal regulating pericyte contractility, but also can cause cell death in the retinal microvasculature. As shown in Fig. 2, this nucleotide activates two potentially lethal pathways (Sugiyama et al. 2005). Both pathways require the activation of P2X7 purinoceptors whose activation not only opens the ligandgated channels, but also can be associated with the formation of large transmembrane pores. These pores, which are permeable to molecules of up to 900 Da, can disrupt cellular metabolism and cause microvascular cell death. In addition to the opening of lethal pores, a cytotoxic response to ATP can occur when a P2X7-induced depolarization activates voltagedependent calcium channels (Fig. 2).
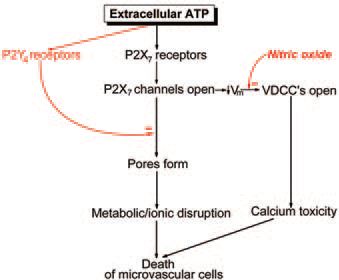
The discovery of the lethal effects of ATP in isolated retinal microvessels raises the possibility that endogenous vasoactive signals can also be vasotoxic. Although the idea that signaling molecules can cause cell death in the circulatory system is new, the concept of extracellular signals having both physiological and pathological effects is not novel. For example, in the nervous system it is well known that the excitatory transmitter glutamate can be neurotoxic. Analogous to excitotoxicity playing a role in neuronal pathobiology, purinergic vasotoxicity may be an important cause for microvascular dysfunction.
Because microvascular cell death has dire consequences for neuronal function, it would seem essential for purinergic vasotoxicity to be prevented in the normal retina. Consistent with this, ATP’s lethality can be held in check by two mechanisms: a P2Y4mediated inhibition of P2X7 pore formation and a nitric oxide-mediated inhibition of voltage-dependent calcium channels (VDCCs) (Fig. 2). A challenge for the future is to determine whether dysfunction of these protective pathways plays a role in sightthreatening microvascular disorders such as diabetic retinopathy.
Acknowledgements
Research in the author’s laboratory is supported by grants from the National Institutes of Health and Research to Prevent Blindness, Inc.
References
Kawamura H, Oku H, Li Q, Sakagami K & Puro DG (2002). Endothelin-induced changes in the physiology of retinal pericytes. Invest Ophthalmol Vis Sci 43, 882-888.
Kawamura H, Sugiyama T, Wu DM, Kobayashi M, Yamanishi S, Katsumura K & Puro DG (2003). ATP: a vasoactive signal in the pericyte-containing microvasculature of the rat retina. J Physiol 551, 787-799.
Kawamura H, Kobayashi M, Li Q, Yamanshi S, Katsumura K, Minami M, Wu DM & Puro DG (2004). Angiotensin-induced effects on the physiology of the pericyte-containing microvasculature of the rat retina. J Physiol 561, 671-683.
Sakagami K, Wu DM & Puro DG (1999). Physiology of rat retinal pericytes: modulation of ion channel activity by serum-derived molecules. J Physiol 521, 637-650.
Schonfleder U, Hofer A, Paul M & Funk RH (1998). In situ observation of living pericytes in rat retinal capillaries. Microvasc Res 56, 22-29.
Sugiyama T, Kobayashi M, Kawamura H, Yamanishi S, Katsumura K & Puro DG. (2005). Regulation of P2X7-induced pored formation and cell death in pericyte-containing retinal microvessels. Am J Physiol Cell Physiol 288, C568-C576
