
Physiology News Magazine
50 years of caveolae – a round-up
It is 50 years since the first microscopic observation of the tiny flask-shaped plasmalemmal invaginations termed caveolae. Only in the last decade, however, with the discovery of the family of caveolin proteins that are integral to these organelles, have we begun to unravel the possible physiological roles of these enigmatic structures
Features
50 years of caveolae – a round-up
It is 50 years since the first microscopic observation of the tiny flask-shaped plasmalemmal invaginations termed caveolae. Only in the last decade, however, with the discovery of the family of caveolin proteins that are integral to these organelles, have we begun to unravel the possible physiological roles of these enigmatic structures
Features
Michael J Taggart
Medicine and Maternal and Fetal Health Research Centre, University of Manchester, UK
https://doi.org/10.36866/pn.58.13
The decade following the second world war proved to be a prolific period for the application of electron microscopic techniques to the analyses of the ultrastructure of tissues and cells. Through such endeavours Pallade (1953) and Yamade (1955) became the first workers to describe an unusual feature of the plasma membrane of endothelial and epithelial cells: they found that the plasmalemma, far from being uniform, often gave the appearance of regular, Ω-shaped invaginations; Yamade termed these structures ‘caveolae intracellulares’ (see Inset). Ever since, the physiological roles of these organelles has perplexed and fascinated in equal measure. What follows is a very brief round-up of this topic but for more extensive background the reader is directed to the reference list at the end of this article.
The early sightings
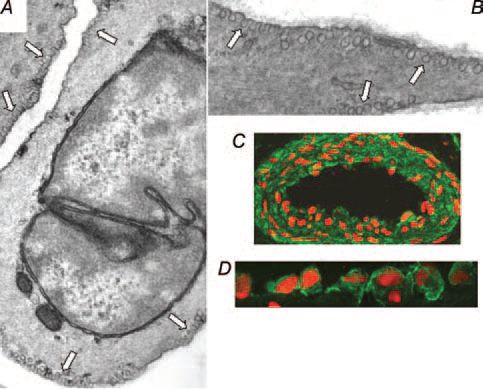
As caveolae were initially discovered in cells lining the lumenal surface of hollow organs, a role in macromolecular transport phenomena was postulated, including capillary permeability and transcytocis and the regulation of cellular free cholesterol flux. With regard to the latter, cholesterol sequestering agents have been known for many years to disrupt the appearance of caveolae. Subsequently, caveolae were found to be prominent, occupying approximately 25% of plasmalemmal surface area, in many other cell types including adipocytes, smooth muscle (Fig. 1) and cardiac cells (with a few notable exceptions being lymphocytes and some cell lines such as HepG2 cells). This wide distribution necessitated consideration of this idea that caveolae might be important for a number of functions. Regulation of transmembrane ion fluxes important for cellular excitability was one possible function ascribed to caveolae in smooth muscle as early as the 1970’s. The observations of a close appositioning of caveolae to elements of the sarcoplasmic reticulum (the major source of releasable organellar Ca2+), and immunogold localisation of a Ca2+-ATPase to caveolae, were instrumental in developing this idea. By and large, however, experimental evidence in support of these different roles of caveolae was observational and progress was hindered by the lack of a definitive molecular marker of caveolar structures.

The missing link identified
The discovery at the turn of the 1990s of a protein component of caveolae, termed caveolin, was to revolutionise the study of these organelles. Caveolin is actually a family of protein molecules of mass 21-24kDa with three main mammalian isoforms (imaginatively termed caveolin-1, -2 and –3). There are two and three isoforms, respectively, of caveolin-1 (α and β) and caveolin-2 (α, β, γ). Whereas these show a wide tissue distribution, caveolin-3 has a much more restricted appearance, being predominant in striated muscle cells. Crucially, transfection of non-caveolincontaining cells, which had no morphological appearance of caveolae, with caveolin-1 (or -3) induced the formation of Ω-shaped invaginations. Subsequently, an interaction of high molecular weight caveolin oligomeric complexes with cholesterol appeared to be key to the formation of caveolae.
Caveolins, however, quickly established themselves as something more than just plasma membranous structural components. Biochemical characterisation studies, including immunoprecipitation, began to highlight a multitude of signalling molecules co-localising with caveolin1. Furthermore, a small 20 amino acid peptide derived from caveolin-1 was, in in vitro assays, found to bind to a whole host of signalling molecules that act downstream of receptor-coupled membrane effectors. In cardiovascular cells, for example, these included PKCα, rhoA, ERK, and nitric oxide synthase (NOS). Binding to this peptide, termed the caveolin scaffolding domain, even altered the enzymatic activity of these signalling molecules and, once introduced into live cells, altered functions as diverse as cytosolmembrane protein translocations, cardiac myocyte beating, flow-induced arterial dilation and eNOS activation.
Much attention focussed on the inhibitory interaction of caveolin and eNOS, regulated by Ca2+-calmodulin and transcriptionally modulated by altering cholesterol levels, and this became something of a model system for understanding other caveolinsignalling molecular events.
Subsequently still more possible functions of caveolin emerged. In endothelial cells, agonist-dependent Ca2+ waves appeared to be initiated at caveolin-rich regions of the plasmalemma, whilst in smooth muscle cells, caveolae disruption with cholesterol-modifying agents altered the appearance of Ca2+ sparks. Both these scenarios supported the earlier suggestions of these organelles contributing to cellular excitability by regulating Ca2+ homeostasis.
Life after caveolae
Just as it seemed that caveolae and caveolins could be all things to all cell biologists and physiologists, a more sobering analysis was demanded following the publication of caveolin knockout mice that were both viable and fertile (cav-1-/-, cav-2-/-, cav-3-/- or cav-1-/-/-3-/-). Clearly, caveolins and caveolae were not essential for life. However, marked phenotypic changes were noticed in cav-/- mice that included elevated triglycerides, cardiomyopathy, changes in pulmonary extracellular matrix and enhanced endothelialdependent relaxation of isolated arteries. Subsequently, the functional implications of caveolin gene depletion has become increasingly clear when the caveolin-/- mice, or cells derived from cav-/- mice, are physiologically challenged. For example, caveolin-1-/mice have a lowered exercise tolerance (probably as a result of the lung histopathologies), a poor response to an insulin tolerance test, impaired angiogenesis, reduced lifespan (due to cardiac hypertrophy and/or pulmonary fibrosis), and aged knockout mice on a high fat diet develop hyperinsulinaemia. Thus the murine caveolin/caveolae ablations, although not lethal, are increasingly providing interesting phenotypic information with relevance to many human pathophysiologies. Indeed, altered caveolin or caveolae levels have now been associated with animal models of hypertension, diabetes, hypercholesterolaemia and heart failure.
Future perspectives
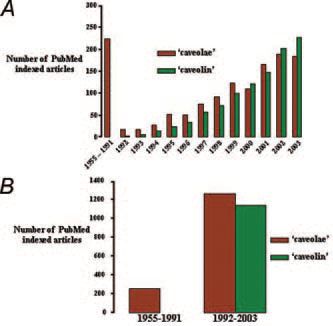
The discovery of caveolins as integral protein components of caveolae has had a huge impact upon the volume of research into these intriguing structures. As illustrated in Fig. 2, as many papers are now published per annum on this topic as in the near-forty years of caveolae research preceding the discovery of caveolin. It is remarkable that many of the initial roles ascribed to caveolae, largely based upon morphological data, have received support from recent molecular studies with caveolins. Yet after 50 years many questions still remain to be answered as to the physiological roles of caveolae – not least of which is the paradox whereby cavolins perform the dual function of being scaffolding molecules for cellular signal integration, but whose binding often exerts an inhibitory regulatory effect. Further consideration of the temporal and spatial dynamics of caveolinregulated signalling will be required to elucidate this and many other puzzles still surrounding these membrane pockets.
References
Feron O & Kelly RA (2001). The caveolar paradox: suppressing, inducing and terminating eNOS signalling. Circ. Res. 88, 129-131.
Taggart MJ (2001). Excitation-contraction coupling in smooth muscle: a role for caveolae and caveolins? NIPS 16, 61-65.
van Deurs B, Roepstorff K, Hommelgaard AM & Sandvig K (2003). Caveolae: anchored, multifunctional platforms in the lipid ocean. Trends Cell Biol. 13, 92-100.
Sonveaux P, Martinive P, DeWever J, et al., (2004) Caveolin-1 expression is critical for vascular endothelial growth factor-induced ischemic hindlimb collateralization and nitric oxide-mediated angiogenesis. Circ Res. 95, 154-161.
Cohen AW, Hnasko R, Schubert W & Lisanti MP. (2004) Role of caveolae and caveolins in health and disease. Physiol Rev. 84, 1341-1379.
