
Physiology News Magazine
Obesity – why all the noise?
Features
Obesity – why all the noise?
Features
Paul Trayhurn
Neuroendocrine & Obesity Biology Unit, University of Liverpool, UK
https://doi.org/10.36866/pn.58.15

It is almost impossible to be unaware of the growing concern with obesity. Newspapers, radio and other media present a steady stream of stories, from the rising tide of the numbers of obese to breaking news of the latest potential targets for a ‘cure.’ Even politicians (the slimmer ones?) have entered the fray, with a recent enquiry from the House of Commons Health Committee calling for UK Government action to stem the rise in obesity. More relevantly, it is also a growing focus in biomedical research with Science, for example, publishing a special issue on the area in February 2003 with a series of articles, an Editorial (The ironic politics of obesity) and a cover featuring fat cells. But why all the attention?
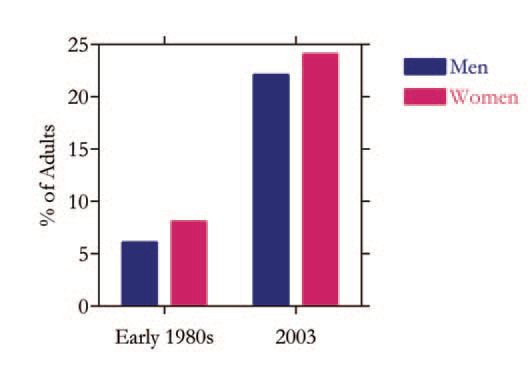
There is no doubt that the incidence of obesity is rising rapidly and some have even described the situation as a ‘pandemic’. The UK provides a stark illustration of the international trends. In the early 1980s just 6% of men and 8% of women in the UK were classified as obese, but the latest figures indicate that the incidence has increased 3-fold in the past 20 years to 22 and 24% of men and women, respectively (Fig. 1). Obesity is customarily defined on the simple basis of body mass index (wt in kg/height in m2), and a value of 30 or more equates to clinical obesity (a lower value is increasingly used in East Asia). There has also been a corresponding increase in overweight (BMI: 25-29.9). Although the UK has one of the highest obesity rates in Europe, the situation is worse in the United States. The focus has until recently been on adults, but there is now growing concern with the rapid rise in overweight and obesity in children.
How much does this really matter? The answer is greatly; being obese reduces life expectancy by on average 8 years, and there is an increased incidence of several major diseases, particularly type 2 diabetes, coronary heart disease and certain cancers (such as breast, colon). Proportionately, the greatest impact of obesity is on type 2 diabetes, the risks for which increase approximately 10fold once the threshold of a BMI of 30 is reached – and the more obese the greater the risk. The consequences of the link between diabetes and obesity are considerable; the current figure of nearly 1.8 million diagnosed diabetics in the UK is predicted to rise to over 3 million within a few years, essentially as a result of the surge in obesity. In the case of cancer, in the UK being obese is now seen as important a risk factor as smoking.
Treating obesity, and indeed reversing the rise in incidence, is in principle simple – either food intake should be reduced or energy expenditure increased. In other words, obesity is fundamentally an issue of energy balance, developing only when intake is in excess of expenditure (Trayhurn, 2005). However, public health messages encouraging dietary change and increased exercise, whether from scientists, clinicians, or Government agencies, have had little impact. Whether direct Government intervention, as increasingly advocated, will be helpful, or counter-productive as some fear, is a moot point. Drugbased approaches to treatment are being actively pursued, and pharmaceutical companies have extensive programmes for the development of new anti-obesity agents, and these are targeted particularly to the suppression of appetite.
The rapid increase in obesity is in practise a reflection of social and cultural changes, principally the now ready access to cheap and palatable high fat foods together with a sedentary lifestyle. What then is the role of biomedical research, and of physiologists in particular, in this area? The central challenge, as it has long been, lies in unravelling the fundamental mechanisms of the regulation of energy balance and body weight. There is an underlying genetic component to obesity, but it is not a genetic disease in the sense of resulting from single gene mutations (with rare exceptions). However, a number of specific gene polymorphisms are associated with weight gain and obesity. In essence, our genetically determined physiological mechanisms for body weight regulation have been overwhelmed by lifestyle changes.
Energy balance has traditionally been considered separately in terms of food intake and the components of energy expenditure, the differences between which are buffered by changes in the storage of triacylglycerols in white adipose tissue. In recent years there have been major developments in our understanding of appetite control. New neuroendocrine factors which either inhibit or stimulate appetite, such as orexin B, cocaine- and amphetamineregulated transcript, and the endogenous cannabinoid system have been identified, adding to established factors such as neuropeptide Y (Wilding, 2002; Trayhurn, 2005). Recently identified peripheral signals include leptin, released largely from adipose tissue, and ghrelin and peptide YY (3-36) from the gut. The challenge is to integrate the nexus of central neuroendocrine pathways and the various peripheral factors into a coherent view of the appetite system.
The main components of energy expenditure are the basal metabolic rate, adaptive thermogenesis (whether from cold, diet, drugs) and physical activity. A continuing theme has been the extent to which adaptations in expenditure leading to the dissipation of excess energy intake as heat are important in the regulation of energy balance and the development of obesity (Trayhurn, 2005). The issue gained resonance with the recognition of brown adipose tissue as the key site of non-shivering thermogenesis in rodents, the tissue generating heat by the controlled uncoupling of oxidative phosphorylation through the presence of the tissue-specific mitochondrial uncoupling protein-1 (UCP1). Contrary to initial expectations, the subsequent discovery of a family of mitochondrial ‘uncoupling proteins’ (UCP2, UCP3…) has not led to the identification of new loci for adaptive thermogenesis (Rousset et al. 2004). Perhaps the most interesting development in energy expenditure is the emergence of the concept of ‘NEAT’ (non-exercise activity thermogenesis) in which small movements (such as fidgeting) play a role in energy balance regulation
(Levine et al. 1999).
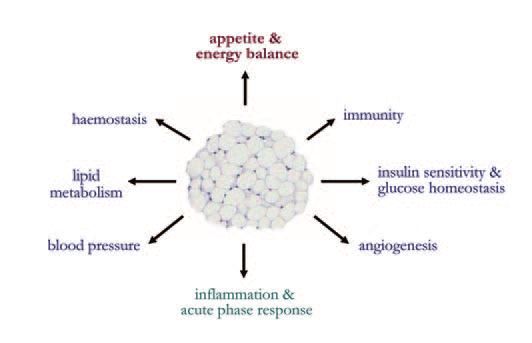
Until recently, white fat was viewed simply as fuel reserve, passively buffering differences between intake and expenditure. However, it is now recognised as a key endocrine organ which plays a central role in energy balance through the secretion of leptin (Zhang et al. 1994). This hormone acts as a powerful satiety factor, interacting with multiple neuroendocrine systems in the hypothalamic control of appetite. In practise, leptin (a pleiotropic hormone) is one of the rapidly expanding list of protein signals secreted by white adipocytes. Indeed, adipocytes are veritable secretory powerhouses, releasing in excess of fifty different hormones and protein factors, termed adipokines (Trayhurn & Beattie, 2001; Trayhurn & Wood, 2004). These adipokines include adiponectin and resistin, which have been the focus of considerable attention because of their putative role in insulin resistance and glucose homeostasis.
The diversity of adipokines is considerable, and their secretion indicates that white adipose tissue communicates extensively with other organs and is involved in a multiplicity of metabolic functions beyond lipid storage (Fig. 2). A number of adipokines are related to inflammation and the inflammatory response, including cytokines and acute phase proteins (Trayhurn & Wood, 2004), and the production of these is generally increased as adipose tissue mass expands in obesity. A key development is the recent recognition that obesity is characterised by chronic low grade inflammation, with adipose tissue being central to this (Trayhurn & Wood, 2004).
Changes in adipokine production in obesity are increasingly considered causal in the development of obesityrelated diseases, particularly type 2 diabetes and the metabolic syndrome. Consequently, there is now the possibility that these associated diseases may be amenable to treatment, whether through pharmacological or nutritional intervention, by targeting specific adipokines. Indeed, there is more cause for optimism with this approach than with overcoming the social and cultural changes which have led to the tide of obesity itself.
References
Levine JA, Eberhardt NL & Jensen MD (1999). Role of nonexercise activity thermogenesis in resistance to fat gain in humans. Science 283, 212-214.
Rousset S, Alves-Guerra MC, Mozo J, Miroux B, Cassard-Doulcier AM, Bouillaud F & Ricquier D (2004). The biology of mitochondrial uncoupling proteins. Diabetes 53 Suppl 1, S130-S135.
Trayhurn P (2005). The biology of obesity. Proc Nutr Soc 64, in press.
Trayhurn P & Beattie JH (2001). Physiological role of adipose tissue: white adipose tissue as an endocrine and secretory organ. Proc Nutr Soc 60, 329-339.
Trayhurn P & Wood IS (2004). Adipokines: Inflammation and the pleiotropic role of white adipose tissue. Br J Nutr 92, 347-355.
Wilding JP (2002). Neuropeptides and appetite control. Diabetic Med 19, 619-627.
Zhang YY, Proenca R, Maffei M, Barone M, Leopold L & Friedman JM (1994). Positional cloning of the mouse obese gene and its human homolog. Nature 372, 425-432.
