
Physiology News Magazine
Impact of the intrauterine environment on respiratory health throughout life
The fetal environment, particularly oxygen and nutrient availability, is critical for organ development. Recent studies show that the lungs can be permanently altered by conditions that restrict fetal growth
Features
Impact of the intrauterine environment on respiratory health throughout life
The fetal environment, particularly oxygen and nutrient availability, is critical for organ development. Recent studies show that the lungs can be permanently altered by conditions that restrict fetal growth
Features
Richard Harding, Megan Cock, & Gert Maritz
Department of Physiology, Monash University, Melbourne, Victoria, Australia
https://doi.org/10.36866/pn.58.19


The concept that the intrauterine environment can have long-term or ‘programming’ effects on organ development, thereby increasing the risk of adult onset diseases, has grown in strength in the last decade. For example, epidemiological studies from a number of countries have shown that low birthweight resulting from intrauterine growth restriction (IUGR) increases the risk of metabolic disorders such as diabetes and obesity in later life. Important aspects of our prenatal environment that have been shown to affect later organ function and health include fetal oxygenation, nutrient availability, the endocrine environment (particularly corticosteroids), as well as exposure to common maternally-used drugs such as nicotine and alcohol. It is now apparent that these perturbations to the prenatal environment have the potential to permanently interfere with genetic programmes of organ development. Of particular interest to our Fetal and Neonatal Research Group at Monash University is the regulation of prenatal lung development and the impact of the intrauterine environment on pulmonary structure and function during postnatal life.
It is now established that the fetal lung is liquid filled and develops in an expanded state, with a low level of inherent recoil. In the fetus, during at least the last third of gestation, a high level of lung expansion is maintained by a combination of continuous, active secretion of ‘lung liquid’ by the pulmonary epithelium, the resistance of the upper airway (mainly the glottis) to the escape of this lung liquid, and by fetal breathing movements (which tend to oppose lung recoil and resist the escape of lung liquid into the fetal pharynx). We now recognise that this high level of lung expansion before birth is critical for normal growth and structural maturation of the lungs. When the degree of lung expansion is reduced for prolonged periods, such as with oligohydramnios (lack of amniotic fluid) or diaphragmatic defects, lung growth is impaired and structural maturation of lung tissue retarded; if severe these can lead to respiratory insufficiency and death after birth. In survivors, the normal development of alveoli never occurs. Thus the physical environment of the fetal lung has a profound and lasting impact on its growth, architecture and functional capacity.
It is now evident that the metabolic and endocrine environment of the fetus can also have a substantial and persistent effect on lung development and postnatal lung structure. Epidemiological and clinical studies have shown that IUGR, which is associated with fetal hypoxaemia, hypoglycaemia and elevated corticosteroid levels, increases the risk of impaired airway function and respiratory illness in later life (Harding et al. 2004). However, the impact of restricted fetal growth on lung structural development is largely unknown. The problem is not insignificant as up to 10% of babies are considered to be growth restricted; that is, they do not reach their expected growth potential in utero. Using sheep, we have restricted fetal growth during the last third of gestation by experimentally inducing placental insufficiency. In this technique, microspheres are administered daily into the fetal arterial supply to the placenta. This embolisation technique induces chronic fetal hypoxaemia, hypoglycaemia and fetal endocrine changes which are similar to responses seen in human IUGR. We have found that restricting fetal growth by placental insufficiency leads to alterations in lung structure, some of which are present at birth, while others develop in the neonatal period (Maritz et al. 2001).
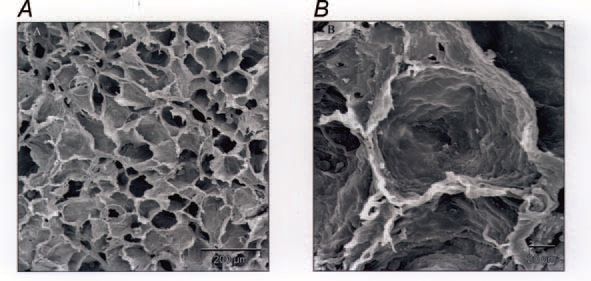
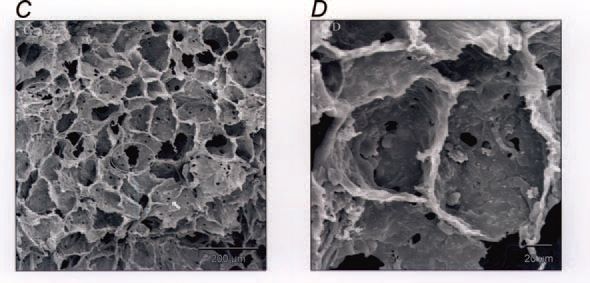
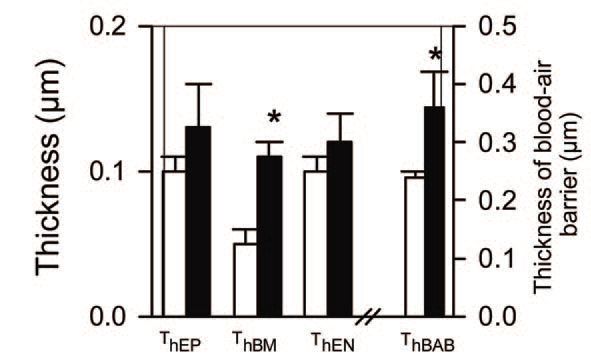
Of considerable interest is the finding that these changes persist at least until maturity at 2.3 years of age (Maritz et al. 2004). In particular, the lungs formed fewer alveoli and the alveoli were larger, resulting in a reduced surface area for gas exchange. In addition, the alveolar blood-air barrier was significantly thicker throughout postnatal life as a result of a thicker basement membrane; together with a reduced alveolar surface area, this would be expected to impair gas transfer. Indeed we observed a reduction in the lung diffusing capacity for CO in the postnatal IUGR animals (Joyce et al. 2001). The alveolar walls were thicker after IUGR due to excessive accumulation of extracellular matrix, which may have contributed to increased lung stiffness in postnatal animals; this increased thickness persisted to maturity. In this regard, it is of interest that the expression of pulmonary surfactant proteins was not altered by IUGR. An unexpected finding was an increased presence of inter-alveolar pores, which is often taken as a sign of aging of the lungs; these pores are regarded as an early sign of emphysematous changes in the lung, as seen with aging or chronic exposure to tobacco smoke. Hence, in the parenchyma of the lung, there was evidence of both impaired development and premature aging. We also observed changes in the airways of these IUGR animals, notably an increased presence of epithelial secretory cells and transiently thinner airway walls
(Wignarajah et al. 2002).
What could be the underlying processes leading to these persistent alterations in lung structure? As elastin is a longlived structural protein that is intimately involved in lung development, including alveolar formation, we have examined the expression of tropoelastin and elastin content in the lungs after fetal growth restriction. Elastin synthesis is known to be metabolically regulated and hence it seemed reasonable to expect it to have been altered by IUGR; however, we found that this was not the case (Cock et al. 2004). At present, it seems likely that FGR leads to persistent alterations in pulmonary extracellular matrix synthesis or degradation in the lung parenchyma.
Maternal cigarette smoking during pregnancy is a major cause of low birthweight, and nicotine exposure has been shown to exert its own effects on the developing lung and airways. Infants and children who were exposed in utero to the effects of maternal smoking are known to be at increased risk of impaired airway function and respiratory illnesses such as asthma. It seems likely that at least part of this smoking-related problem is due to IUGR, and that the effects remain to adulthood. However, this remains to be established.
In summary, our studies in sheep have shown that physical, metabolic and endocrine impairments in the intrauterine environment can alter lung development in the fetus, and that these changes can persist to maturity. Therefore, in fully understanding adult respiratory illness, prenatal environmental factors must be taken into account. It is also clear that to enable our progeny to have the best possible respiratory function throughout their lives, as parents we must ensure that they have the best possible intrauterine experience.
This article is based on ‘The lung from fetus to neonate: impact of the intrauterine environment’ presented at the IUPS in 2001.
References
Cock ML, Joyce BJ, Hooper SB, Wallace MJ, Gagnon R, Brace RA, Louey S & Harding R (2004). Pulmonary elastin synthesis and deposition in developing and mature sheep: effects of intrauterine growth restriction. Exp Lung Res 30, 405-418.
Harding R, Albuquerque CA & Cock ML (2004). Role of nutrition in lung development before and after birth. In The Lung: Development, Aging and the Environment, ed. Harding R, Pinkerton KE & Plopper CG, pp 253-266. Elsevier, London.
Joyce BJ, Louey S, Davey MG, Cock ML, Hooper SB & Harding R (2001). Compromised respiratory function in postnatal lambs following placental insufficiency and intrauterine growth restriction. Ped Res 28, 931-937.
Maritz GS, Cock ML, Louey S, Joyce BJ, Suzuki K & Harding R (2004). Fetal growth restriction has long-term effects on postnatal lung structure. Ped Res 55, 287-295.
Maritz GS, Tester ML, Louey S, Joyce BJ, Albuquerque CA & Harding R (2001). Effects of fetal growth restriction on lung development before and after birth: a morphometric analysis. Ped Pulmonol 32, 201-210.
Wignarajah D, Cock ML, Pinkerton KE & Harding R (2002). Influence of intrauterine growth restriction on airway development in fetal and postnatal sheep. Ped Res 51, 681-688.
