
Physiology News Magazine
Mechanosensory transduction in the enteric nervous system
Two classes of sensory neuron within the myenteric plexus of the colon give complementary information regarding the tension of smooth muscle and its degree of stretch. Both sensory systems appear to be capable of generating a different pattern of motility
Features
Mechanosensory transduction in the enteric nervous system
Two classes of sensory neuron within the myenteric plexus of the colon give complementary information regarding the tension of smooth muscle and its degree of stretch. Both sensory systems appear to be capable of generating a different pattern of motility
Features
Terence K Smith, Nick J Spencer, & Grant W Hennig
Department of Physiology and Cell Biology, University of Nevada School of Medicine, Reno, NV, USA
https://doi.org/10.36866/pn.58.23

Over the last few years the intriguing similarities and differences between the simple reflex behaviour of the somatic nervous system and the enteric nervous system (ENS) are gradually emerging. We have presented evidence that there are two distinct sensory systems in the ENS that underlie muscle movements of the gut wall that drive fecal pellet propulsion. Mucosally projecting AH neurons appear to register the tension (tone) of smooth muscle, whereas S interneurones register the length of smooth muscle or gut diameter. Functionally, these sensory modalities appear analogous to those in skeletal muscle where Golgi tendon organs and muscle spindles within the same muscle bundle give complementary information about changes in muscle force and length respectively.
The ENS lies within the intestinal wall and consists of two ganglionated neural networks – these are the myenteric and submucous plexuses, that mainly regulate motility and secretion respectively. Stretching of the gut wall or mucosal stimulation usually elicits simultaneous contraction of the longitudinal (LM) and circular (CM) smooth muscle orally and relaxation of both smooth muscles anally. These reflex responses underlie propulsion of gut contents or peristalsis (Bayliss & Starling, 1899).
The neurons involved in these reflex pathways lie within the myenteric plexus and have been classified into
S/Type I and AH/Type II neurons (Hirst et al. 1974). S/Type I neurons receive extensive fast excitatory synaptic (S) input. They are slowly adapting neurons that comprise excitatory and inhibitory motor neurons and interneurons. In keeping with their function, S-neurons respond to distension or mucosal stimulation with bursts of fast excitatory postsynaptic potentials (fEPSPs) (Smith et al. 1992). AH/Type II neurons, on the other hand, are characterized by a prolonged afterhyperpolarization (AH-up to 20s) that follows a single action potential. They receive little fast synaptic input but can generate slow excitatory postsynaptic potentials (sEPSPs) in other AH neurons and S neurons.
AH-neurons, unlike S neurons, do not respond to reflex stimulation when it is applied some distance away from the recording site (Smith et al. 1992; Spencer & Smith, 2004). AH neurons are multipolar neurons with one or more processes projecting down into the intestinal mucosa. Unlike S neurons, they respond directly to chemical stimulants applied to the mucosa. AH-neurons also respond to stretch with an ongoing action potential discharge. Surprisingly, this discharge is dependent upon muscle tone/tension rather than stretch per se, since despite maintained stretch, their activity is abolished by drugs such as nicardipine (L-type Ca2+ channel antagonist) and isoprenaline (β-antagonist) that abolish smooth muscle tone (tone being the muscles capability of generating active tension to resist stretch) (Kunze et al. 1998).
As a result of these findings, it has been assumed until now that myenteric AHneurons are the only intrinsic primary afferent neurons in the gut that are responsible for initiating the peristaltic reflex. However, our earlier studies suggested that enteric reflexes activated by mucosal stimulation and stretch are mediated by two different sensory neurons that converge onto common interneurons and motor neurons within the reflex pathways (Smith et al. 1992). Previously we have shown interactions between these two sensory systems; habituation of the response to repetitive stretch can be overcome, and even sensitized, by a preceding mucosal stimulus (Smith et al. 1991). Although it seems likely that mucosal reflexes are initiated by AH neurons, the intrinsic sensory neurons mediating stretch reflexes have only recently been identified (Spencer & Smith, 2004). We show below that these different sensory neurons can produce different motor behaviours, depending upon the stretch and tone of the smooth muscle.
Muscle tone dependent peristalsis
We investigated the relationship between smooth muscle tone and propulsion of fecal pellets in the guinea-pig distal colon (Smith et al. 2003). This was in part to determine whether smooth muscle tone-dependent enteric neurons may contribute to peristalsis. To do this we threaded a segment of distal colon through two partitions, which divided the bowel for pharmacological purposes into oral, stimulation and anal regions. An intraluminal balloon to mimic a fecal pellet was inserted between the partitions (stimulation chamber) and held in position. Maintained distension of the balloon produced rhythmic (~0.3/min), peristaltic-like waves of contraction that propagated down the colon. Each wave of contraction (duration ~40-60s) exerted considerable force on the balloon. These waves were neural in origin since they were blocked by hexamethonium. When a smooth muscle relaxant (isoproterenol, nicardipine or papavarine) was added selectively to the stimulation chamber the muscle relaxed and the peristalticlike waves were abolished. Atropine, a muscarinic antagonist, added to the stimulation chamber also relaxed the muscle and blocked peristaltic waves. This suggested that ongoing cholinergic excitatory motor nerve activity was largely responsible for generating smooth muscle tone around the balloon. Therefore, the enteric neural circuitry responsible for these waves was critically dependent upon smooth muscle tone. In addition, removing the mucosa around the balloon also abolished the rhythmic peristaltic waves. Thus it seems likely that smooth muscle tone dependent AH neurons may be involved in initiating these waves.
Ongoing stretch-activated, muscle tone-independent, reflex activity
We also made simultaneous intracellular electrical recordings from the CM or LM at either end of a sheet of guinea-pig distal colon that was maintained under circumferential stretch (Spencer et al. 2002, 2003). At the oral end of these stretched sheet perparations excitatory junction potentials (EJPs) occurred at the same time in both the LM and CM. If an oral EJP elicited an action potential it evoked a robust contraction that propagated anally. At the anal end of the tissue inhibitory junction potentials (IJPs) occurred synchronously in both muscle layers. Also, the rapidly firing (frequency of ~15/min) oral EJPs were synchronized in both time and amplitude with the anal IJPs recorded some 20mm away (Fig. 1). Unstretched tissues did not exhibit this activity. Unlike the muscle tone dependent peristaltic waves, ongoing reflex activity was unaffected by removal of the mucosa and the submucous plexus. This rhythmic motor pattern was also unaffected by abolishing smooth muscle tone in the stretched segment of distal colon with nifedipine (L-type channel antagonist that blocks smooth muscle action potentials).
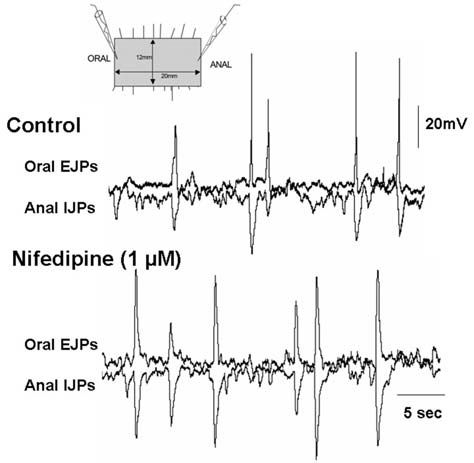
The only way we could envisage the oral EJPs and anal IJPs to be ‘locked’ in both time and amplitude and to occur at the same time in both the LM and CM muscles (see Fig. 1) was if the excitatory and inhibitory motor neurons innervating both muscles at either end of the tissue were activated by common interneurons in ascending excitatory and descending inhibitory nerve pathways that communicate with one another (see Fig. 2). Taken together, these results suggested that muscle-tone dependent activity in AH neurons was unlikely to drive this ongoing reflex activity. It seemed more likely that only S neurons participated in this ongoing motor pattern.

Mechanosensitive interneurons and S motor neurons
We next attempted to determine whether S or AH neurons were involved in driving this muscle toneindependent, stretch-activated, ongoing reflex activity. To do this we made simultaneous intracellular electrical recordings from both myenteric neurons and adjacent CM cells in stretched sheets of distal colon (Spencer & Smith, 2004). AH neurons were found to be electrically silent despite ongoing junction potentials in the muscle. S motor neurons, on the other hand, showed phasic bursts of fEPSPs that just preceded an EJP or IJP in the muscle. Another class of S neurons exhibited a continuous high frequency burst of action potentials. This ongoing discharge was insensitive to synaptic blockade with low Ca2+/high Mg2+ solution, which blocked the coordinated EJPs and IJPs in the muscle. The discharge of action potentials in these neurons could often be converted to proximal process potentials by membrane hyperpolarization.
We therefore assumed this activity was generated by a mechano-sensitive soma or stretch sensitive dendrites. This was confirmed since, even after synaptic blockade, stretching the adjacent CM with a fine probe produced an increased discharge of action potentials in these neurons. Dye injection revealed that these particular neurons had a filamentous soma and were ascending and descending interneurons since their long axon gave off collateral branches to other neurons as it passed through several myenteric ganglia. Interestingly, a dendrite could usually be traced to leave the underside of a ganglion and enter the CM where it ran parallel to the CM fibres. Dendrites from these neurons did not appear to enter the LM. Therefore, it appears that these dendritic processes within the CM are involved in stretch activation of these mechanosensitive interneurons. This was supported by the observation that removing the LM had no effect on the ongoing reflex activity. However, no activity was recorded in the LM of stretched sheets devoid of CM. It turns out that mechanosensory interneurons are not unusual since they have been identified in a variety of invertebrates (reviewed in Spencer & Smith, 2004).
Conclusions and future directions
We have found two different neurallymediated motor behaviours in the guinea-pig distal colon that likely underlie fecal pellet propulsion: smooth muscle tone-dependent peristaltic waves and tone-independent, stretchactivated ongoing reflex activity. Stretch-sensitive S interneurons are the only sensory neuron needed to drive ongoing reflex activity. However, peristaltic waves, which require stretch, smooth muscle tone and mucosal stimulation for their activation, likely involve a complex interaction between AH sensory neurons and stretchactivated S interneurons. Presumably, there is a positive feedback when both sensory neurons are activated. Activity in AH neurons could increase the excitability of interneurons by inducing sEPSPs. Oral contraction activated by interneurons could further excite tonedependent AH neurons.
Future directions will include determining how these two sensory systems interact to produce intestinal propulsion. By analogy with Golgi tendon organs and muscle spindles within the skeletal muscular system, we will also need to determine what in series and in parallel elements are responsible for transducing muscle tension and muscle stretch to AH and S interneurons.
A possibility is that the intramuscular interstitial cells of Cajal (ICC-IM), which run parallel to and within CM bundles, initiate stretch-dependent activity in the mechanosensitive dendrites of S interneurons. ICC-IM also mediate excitatory and inhibitory neuro-transmission from motor neurons to the muscle (Beckett et al. 2004).
Acknowledgements
We thank our past students that have participated in these experiments. We also thank the National Institute of Health USA (NIDDK R01 45713) for their generous support.
References
Bayliss WM & Starling EH (1899). The movements and innervation of the small intestine. J Physiol 24, 99–143.
Beckett EA, Bayguinov YR, Sanders KM, Ward SM & Hirst GD (2004). Properties of unitary potentials generated by intramuscular interstitial cells of Cajal in the murine and guinea-pig gastric fundus. J Physiol. 559: 259-69.
Hirst GDS, Holman ME & Spence I (1974). Two types of neurons in the myenteric plexus of duodenum in the guinea-pig. J Physiol 236, 303-326.
Kunze WAA, Furness JB, Bertrand PP & Bornstein JC (1998). Intracellular recording from myenteric neurons of the guinea-pig ileum that respond to stretch. J Physiol 506, 827–842.
Smith TK, Bornstein JC & Furness JB (1991). Interactions between reflexes evoked by distension and mucosal stimulation: electrophysiological studies of guinea-pig ileum. J Auton Nerv Syst 34, 69–75.
Smith TK, Bornstein JC & Furness JB (1992). Convergence of reflex pathways excited by distension and mechanical stimulation of the mucosa onto the same myenteric neurons of the guinea-pig small intestine. J Neurosci 12, 1502–1510.
Smith TK, Oliver GR, Hennig GW, O’Shea D, Vanden Berghe P, Kang SK & Spencer NJ (2003). A smooth muscle tone-dependent migrating motor pattern in guinea-pig distal colon. J Physiol 551, 955–969.
Spencer NJ, Hennig GW & Smith TK (2002). A rhythmic motor pattern activated by circumferential stretch in guinea-pig distal colon. J Physiol 545, 629–648.
Spencer NJ, Hennig GW & Smith TK (2003). Stretch activated neuronal pathways in longitudinal and circular muscle of guinea-pig distal colon. Am J Physiol 284, G231–G241.
Spencer NJ & Smith TK (2004). Mechanosensory S-neurons rather than AH-neurons appear to generate a rhythmic motor pattern in guinea-pig distal colon. J Physiol 558, 577-596.
