
Physiology News Magazine
Myocardial connexin 43: gap junction-dependent and gap junction-independent effects on ischemia/reperfusion injury
Everyone knows the heart is a ‘functional syncytium’ with adjacent cells electrically coupled by connexin 43-formed intercellular channels (gap junctions). But these connexin 43 molecules may have more subtle roles in ischemia – reperfusion injury
Features
Myocardial connexin 43: gap junction-dependent and gap junction-independent effects on ischemia/reperfusion injury
Everyone knows the heart is a ‘functional syncytium’ with adjacent cells electrically coupled by connexin 43-formed intercellular channels (gap junctions). But these connexin 43 molecules may have more subtle roles in ischemia – reperfusion injury
Features
Antonio Rodriguez-Sinovas, David García-Dorado, Alberto Cabestrero, & Marisol Ruiz-Meana
Laboratorio de Investigación Cardiovascular, Servicio de Cardiología, Hospitals Vall d’Hebron, Barcelona, Spain
https://doi.org/10.36866/pn.58.31

Connexin 43 (Cx43) is the main protein forming gap junctional channels in mammalian cardiomyocytes. Six Cx43 molecules form a hemi-channel (connexon) that docks to another hemichannel in the plasma membrane of an adjacent cell to assemble a complete junctional channel. These channels allow cell-to-cell passage of molecules of less of 1 kDa, including ions and most second messengers. This results in electrical and chemical coupling of adjacent cardiomyocytes, essential for normal heart function. Altered electrical coupling through gap junction channels has been associated with arrhythmogenesis in different pathological conditions.
Previous studies demonstrated that after ischemia/reperfusion, dead, hypercontracted cells are not found scattered across the myocardium, but are connected to other dead myocytes within well-delimited areas of contraction band necrosis, a pattern that could be explained only by the existence of some kind of interaction between cells. The first evidence suggesting that this interaction could be chemical and mediated through gap junctions came from studies using heptanol, a gap junction uncoupler, that was able to reduce infarct size and LDH release in two different models of ischemia/reperfusion (Garcia-Dorado et al. 1997). However, the therapeutic potential of the protective effect of inhibition of gap junction communication against reperfusion injury is limited by the low specificity of heptanol and by the undesirable effects of such inhibition in normal myocardium. For these reasons, we have recently extended our studies to three other chemically unrelated gap junction uncouplers. We have correlated their protective effects on infarct size with their effects on the recovery of macroscopic electrical properties of the myocardium (i.e. tissue resistivity), an indirect marker of gap junction closure (Rodriguez-Sinovas et al. 2004). We found that gap junction uncoupling with heptanol, 18α-glycyrrhetinic acid, palmitoleic acid or halothane reduced reperfusion injury, as assessed by LDH release, in isolated rat hearts, and that these effects correlated consistently with an attenuation in the recovery of myocardial electrical resistivity during reperfusion. More importantly, these effects occurred at concentrations that lacked any measurable effect on myocardial electrical resistivity in normal hearts during normoxia. This opens the possibility of developing new therapeutic strategies that selectively interfere with gap junction mediated spread of necrosis in myocardium undergoing reperfusion, but which have minimal actions on macroscopic electrical properties in the myocardium at distance.
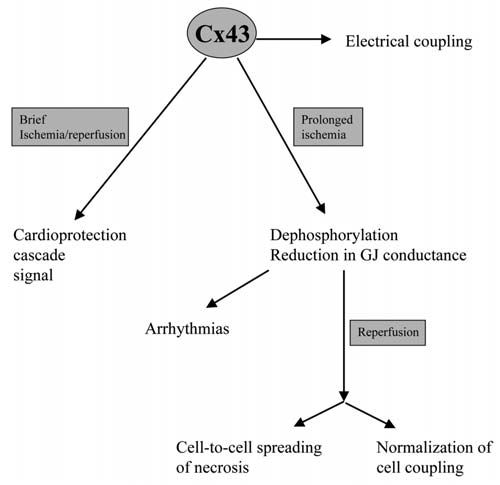
Propagation of cell injury through gap junction channels is not exclussive to the myocardium, and has been also described in other tissues such as astrocytes (Lin et al. 1998), and during chemical or physical treatment of tumour cells (Azzam et al. 2001). However, gap junctions play a complex role in cell death and their effects may vary depending on the conditions. In fact, it has been reported that they can reduce the susceptibility of rat neonatal myocardial cells to potentially lethal insults by diluting them within a larger cell mass (Yasui et al. 2000).
An intriguing point is the role that Cx43 may play during ischemic preconditioning, a phenomenon by which brief episodes of ischemia/reperfusion protect the myocardium from the damage induced by a subsequent more prolongued ischemia. Heptanol administered before preconditioning ischemia attenuated the protective effect in isolated mouse hearts (Li et al. 2002), and underexpression of Cx43 in a transgenic mouse model completely abolished the preconditioning protection (Schwanke et al. 2002).
However, this effect seems to be independent of gap junctional communication, since it is maintained in isolated cardiomyocytes from heterozygous Cx43-deficient mice (Li et al. 2004). Gap junctionalindependent effects of Cx43 have been described in other cell types, notably astrocytes, in which forced expression of this protein was protective against energy depletion despite physical separation of the cells (Lin et al. 2003).
The possibility that Cx43 may play a role ischemic preconditioning independently of gap junctional communication opens a new area of research in the field of cardiac protection.
Acknowledgements
This work was partially supported by grants FIS 01/3135, SAF 2002-00759, and Redes Tematicas de Investigacion Cooperativa (RECAVA, C03/01).
References
Azzam, E. I., de Toledo, S. M., & Little, J. B. (2001). Direct evidence for the participation of gap junction-mediated intercellular communication in the transmission of damage signals from alpha particle irradiated to nonirradiated cells. Proc Natl Acad Sci U.S.A 98, 473-478.
Garcia-Dorado, D., Inserte, J., Ruiz-Meana, M., Gonzalez, M. A., Solares, J., Julia, M., Barrabes, J. A., & Soler-Soler, J. (1997). Gap junction uncoupler heptanol prevents cell-to-cell progression of hypercontracture and limits necrosis during myocardial reperfusion. Circulation 96, 3579-3586.
Li, G., Whittaker, P., Yao, M., Kloner, R. A., & Przyklenk, K. (2002). The gap junction uncoupler heptanol abrogates infarct size reduction with preconditioning in mouse hearts. Cardiovasc Pathol 11, 158165.
Li, X., Heinzel, F. R., Boengler, K., Schulz, R., & Heusch, G. (2004). Role of connexin 43 in ischemic preconditioning does not involve intercellular communication through gap junctions. J Mol.Cell Cardiol. 36, 161-163.
Lin, J. H., Weigel, H., Cotrina, M. L., Liu, S., Bueno, E., Hansen, A. J., Hansen, T. W., Goldman, S., & Nedergaard, M. (1998). Gapjunction-mediated propagation and amplification of cell injury. Nat.Neurosci. 1, 494-500.
Lin, J. H., Yang, J., Liu, S., Takano, T., Wang, X., Gao, Q., Willecke, K., & Nedergaard, M. (2003). Connexin mediates gap junctionindependent resistance to cellular injury. J Neurosci. 23, 430-441.
Rodriguez-Sinovas, A., Garcia-Dorado, D., Ruiz-Meana, M., & Soler-Soler, J. (2004). Enhanced effect of gap junction uncouplers on macroscopic electrical properties of reperfused myocardium. J.Physiol 559, 245-257.
Schwanke, U., Konietzka, I., Duschin, A., Li, X., Schulz, R., & Heusch, G. (2002). No ischemic preconditioning in heterozygous connexin43-deficient mice. Am.J Physiol Heart Circ.Physiol 283, H1740-H1742.
Yasui, K., Kada, K., Hojo, M., Lee, J. K., Kamiya, K., Toyama, J., Opthof, T., & Kodama, I. (2000). Cell-to-cell interaction prevents cell death in cultured neonatal rat ventricular myocytes. Cardiovasc Res. 48, 68-76.
