
Physiology News Magazine
Understanding standing
Standing is something we do without conscious thought, but it is a complex task involving the nervous system, muscles and tendon properties - and anticipation! Martin Lakie and Ian Loram explain
Features
Understanding standing
Standing is something we do without conscious thought, but it is a complex task involving the nervous system, muscles and tendon properties - and anticipation! Martin Lakie and Ian Loram explain
Features
Martin Lakie & Ian Loram
School of Sport and Exercise Sciences, University of Birmingham, Birmingham, UK
https://doi.org/10.36866/pn.57.19

In standing the body’s centre of mass (the imaginary point at which the mass of the body can be considered to be concentrated, approximately in the small of the back) is usually slightly forward of the ankle joint which forms the axle on which the body rotates (Fig. 1A). So it is literally true to say that we are all inclined to fall on our faces. Why does this catastrophe not occur? The answer is that calf muscles, gastrocnemius and soleus are responsible (Fig. 1B). Gravity pulls us down and consequently forward and these muscles pull us up and consequently back. The more we lean forward the harder they have to work. Studies have shown that for most people the angle of forward lean is about 3 degrees. At this angle the mean force in the calf muscles of each leg is about 500 N, which is about 12% of the maximal force that can be produced by a single soleus muscle (figures from Hoy et al. 1990). Due to the muscular activity the total metabolic energy needed to stand is about 25 W greater than for lying supine (Davidson & Passmore, 1966). Although the anatomy seems simple, the problem that has intrigued neurophysiologists for many years is precisely how standing people unconsciously regulate the activity of the calf muscles in response to the gravitational requirement. *1
*1: For simplicity, we mention only forward sway (falls) in this article. Backward sway (throws) are an identical process, with the signs of the movements reversed.

The inverted pendulum
In attempts to answer this question, researchers have used a simplified model to represent human standing. The concept is shown in Fig. 1B. At the outset, it must be admitted that this model does not preserve all the normal features of standing. An assumption is made that all the motion occurs solely at the ankles – i.e. there is no other movement of the limbs or between different segments of the body. Also, it is usual to consider movement in only the forward – back (antero-posterior) plane whereas there is also the problem of side-to-side motion of the body to be considered. Furthermore, the model assumes that the two ankles share a common axis. Observation will show that this is not the way that most people stand. Nevertheless, since its introduction by Smith in 1957 (Smith was a lecturer in Anatomy at the University of St Andrews) the inverted pendulum concept has been a powerful stimulus to explaining how standing works. It is a good example of a reductionist approach where, in order to understand a complicated system, we may remove some of its complex features and study a simplified version. Many studies have confirmed that the fundamental problem in standing is balancing an inverted pendulum. Although human standing is something that we mainly take for granted it is a complex activity that takes all of us about a year of life to accomplish. It is a reasonable hope that by understanding how the inverted pendulum is balanced we will be able to understand howstanding in particular, and other aspects of postural maintenance in general, are controlled.
Sounding sway – a new technique for observing muscles in action
A number of measurements have conventionally been made on standing subjects in attempts to understand the standing process. It is universally agreed that in standing the body is not static. The human inverted pendulum is inherently unstable and small slow irregular sways are continually observed. Thus, body angle, torque and EMG continually fluctuate. It is comparatively easy to measure the muscle forces (or more precisely their close relative the ankle torques). With a little more difficulty the change in body angle can be measured. As far as the muscles themselves are concerned it has previously only been possible to record their EMG. This enables one to see how their activity is varying, but it does not let one see what they are actually doing In this article we describe a new technique which allows us to observe the tiny movements of calf muscles in standing subjects. The new information obtained by this technique suggests that many current ideas about standing require revision.
Our approach to the problem has been the use of dynamic ultrasonography with computerized image analysis
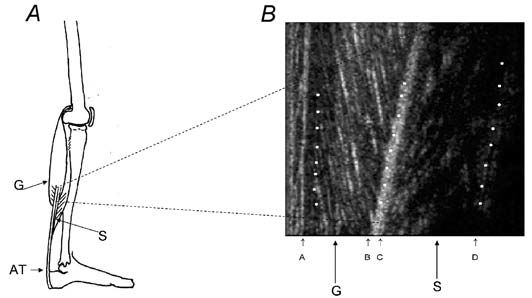
(Loram et al. 2004). The work was done in collaboration with Constantinos Maganaris at Manchester Metropolitan University. Ultrasound is strongly reflected by collagen fibres. The collagen in tendons shows up clearly and collagen also demarcates many of the muscle fibres. This technique has previously been quite extensively used to make static measurements of muscle and tendon lengths. The pointwise resolution of the technique depends on a number of factors including the frequency of the ultrasound and is typically about 0.5 mm.
However, the ultrasound image has the properties of an array of detectors. This considerably increases the resolving power of the technique. As a simple analogy the human eye will struggle to see a dot of 10 mm diameter at 6 metres range – stretch the dot out into a long line 10 mm wide (power cable) and it may be seen at several kilometers. We have used computerized image analysis to take advantage of this fact. It is possible to resolve muscle fibre length changes of as little as 10 µm in the calf. A typical static image of the calf muscles is shown in Fig. 2.
What might happen in standing – orthodox views
Consider Fig. 1B. As the body sways forwards the muscles are stretched. Ankle torque (muscle force) rises (it has to or you fall down). One school of thought (e.g. Winter et al. 2001) has long maintained that the active muscles produce the force automatically as a direct consequence of the stretch because they have spring-like properties. If the spring stiffness is adequate, the force that can be generated by muscle stretch will suffice to prevent a fall. The job of the nervous system is simply to set the resistance to stretch of the muscles to a sufficiently high value; then no further neural intervention is needed. This mechanical tonus theory could be stated as ‘increased force is generated by muscle stretch’. Others have maintained that the mechanical tone of the muscles is inadequate to produce stability. They also observe that forward sway is associated with an increased EMG in the muscles (e.g. Morasso & Sanguineti, 2001; Fitzpatrick et al. 1994). Accordingly, they suggest that stretching the active muscle will generate stretch reflexes that raise tone by increasing activation of the stretched muscle. This reflex tonus view, summarised by the Sherrington School in the ‘little red book’ (Creed et al. 1932), is that ‘increased force is produced by muscle stretch plus reflex activity’. Both theories assume that the muscle will be lengthened by forward sway.
What does happen in standing – paradoxical muscle movements revealed by ultrasonography
However, Fig. 3 shows that forward sway is in fact associated with a shortening of the muscle! Torque rises, EMG increases but the muscle shortens The rise in force cannot be due to muscle tone or muscle reflexes. The muscles and the load generally move in opposite directions.
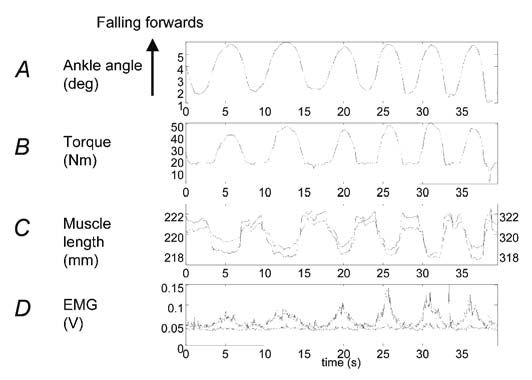
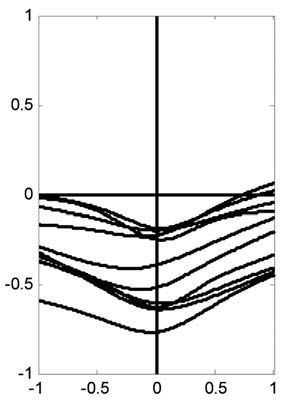
This might at first seem not just counterintuitive, but quite impossible. How can it happen? The answer is that the stiffness of the Achilles’ tendon and foot is not very great at the low forces that are involved in standing. (It is much greater for the large forces involved in running and jumping.) The tendon and the foot together form a spring-like buffer that decouples the muscle and the load (the body). This spring stiffness is not itself sufficient to permit standing. In direct measurements using a piezoelectric stretcher, we have measured the overall stiffness of the ankle. That is, we have abruptly stretched the series combination of the foot, the tendon and the muscle and measured the resulting force increment. This combination (inevitably limited by the weakest link) defines the intrinsic stiffness of the ankle. It represents the intrinsic stiffness because the value is obtained before the nervous system has time to alter the state of the muscle. For 10 subjects the average intrinsic ankle stiffness was only 91% of that necessary for standing (Loram & Lakie, 2002a). Using rather bigger stretches, Morasso’s group (Casadio et al. 2004) have recently obtained an even lower average value of 64%. What this means in effect is that if the muscle remained stationary then stretch of the foot and tendon caused by a sway would generate only 64-91% of the force that is necessary to prevent falling. In order to supply the deficiency, additional stretch of the tendon and foot must be generated. This can only be produced by active shortening of the muscle. Thus, forward sway of the body stretches the tendon – foot spring. Muscle shortening also simultaneously stretches it. These two features acting in concert produce the necessary force for standing. Muscle movement is absolutely necessary and the job cannot be done statically. Figure 3 was obtained from a subject who was voluntarily making large sways. The same process has been observed in subjects standing normally. The body and muscle movements are naturally much smaller but they are clearly on average in the opposite sense (Fig. 4). We have called this process of control by active alterations in muscle length the ballistic bias mechanism (Loram & Lakie, 2002b).
It might seem that the shortfall in stiffness that must be made up by this mechanism is only 9-36%. However, the 100% figure applies to sways that are of infinite duration. For sways that take the times commonly observed in standing (usually ~0.8 s for a unidirectional sway) calculation and experiment suggest that a value of close to 200% is necessary (this figure represents what is often called the effective stiffness). Thus, the intrinsic stiffness of the ankle and the ballistic bias mechanism make a quantitatively approximately equal contribution to the effective stiffness. The intrinsic stiffness almost cancels the force due to gravity and the fine tuning is done by the active process. This probably means that the job of the nervous system is made easier.
Ballistic bias in action – acting on impulse
In an attempt to demonstrate the ballistic bias mechanism we have described some simple experiments in which subjects balance a large inverted pendulum by hand (Lakie et al. 2003). The pendulum represents the body. The hand represents the calf muscles. The hand is connected to the pendulum by a steel spring which defines the intrinsic stiffness. It can be set to any desired value. With values ~70-100% subjects can easily balance the pendulum by active hand movements although none of them were able to describe exactly how they did it. Analysis clearly showed that on average the hand and pendulum moved in opposite directions. However, at any instant the movements are not an exact mirror image. Like the body, the pendulum sways rather slowly with an average duration between turning points of ~0.8 s. The hand movements are faster and intermittent (occurring approximately every 0.3 s). Each hand movement is a ballistic bias adjustment (impulse).
The most basic behaviour of such a system would be a form of oscillation where each impulse violently catches and reverses a fall, throwing the pendulum transiently more upright in the manner of someone balancing on a pogo stick or keeping a tennis ball in the air with a racquet. In reality, depending on the complexity of the task and the sensory information available to the subject, there are generally 2-4 bias adjustments per unidirectional sway. The impulses are smaller and more sophisticated than those made on a pogo stick – consequently they are not all successful (they may merely slow the pendulum but not reverse it).
This is a good strategy because by making the adjustments as small as possible the acceleration, and hence sway size, of the pendulum is minimized. The large inertia of the pendulum buys time for the process to be tried again. The duration of the pendulum sway can therefore be explained as a consequence of an intermittent impulsive discrete motor act taking about 0.3 s and usually needing repetition one or more times per pendulum sway in order to reverse it. It also suggests a plausible mechanism by which any preferred standing position may be preserved. The pendulum or body is moved to, and maintained at, a new position by a series of nudges. As the impulses involve active muscle length changes which are on average in the opposite direction to those that would be passively produced, they must be driven by a signal which anticipates pendulum movement. Muscle activity cannot be a simple reaction to muscle length or tension (if it were it would produce bi-stable or ‘toggle’ action in the muscle). Ballistic bias requires a higher level of control than has commonly been assumed for standing. We have recently been able to observe these ballistic bias adjustments of the calf muscles in normally standing subjects. They are very much smaller than the hand movements, but they have remarkably similar features.
Conclusion
Conventional views assume that the increased force that is required to prevent collapse is produced by stretching the active muscles. In opposition to this view we propose that the force is associated with active shortening of the muscles.
Furthermore, this is not a continuous type of feedback control arising from the muscles, but an impulsive controller which acts intermittently and anticipatorily. Many years ago, Craik showed that individually judged outputs of the nervous system could only be made at a low rate of 2-3 per second (Craik, 1947). The requirement for an anticipatory controller suggests a level of sophistication that is greater than the simple mechanical/reflex ideas that have been commonly proposed.
The place of standing in the hierarchy of motor control requires revision. In standing, cause and effect may have been confused; postural sway is actually generated by anticipatory neural activity whereas it has previously been assumed that neural activity is a reaction to postural sway.
Also, these observations suggest that the behaviour of muscles in other postural tasks assumed to be reflex should be investigated. ‘Static’ postural control may be a myth.
Acknowledgements
IDL is supported by a grant from the Leverhulme Trust.
References
Casadio M, Morasso PG & Sanguineti V (2004). Direct measurement of ankle stiffness during quiet standing: implications for control modeling and clinical application. Posture and Gait (in press).
Craik KJW (1947). Theory of the human operator in control systems I and II. Br J Psychol 38, 56-71 and 142-148.
Creed RS, Denny-Brown D, Eccles JC, Liddell EGT & Sherrington CS (1932). Reflex activity of the spinal cord. Clarendon Press, Oxford.
Davidson S &Passmore R (1966). Human nutrition and dietetics, 3rd edn. E & S Livingstone, Edinburgh & London.
Fitzpatrick RC, Gorman RB, Burke D & Gandevia SC (1994). Loop gain of reflexes controlling human standing measured with the use of postural and vestibular disturbances. J Neurophysiol 76, 3994-4008.
Hoy MG, Zajac FE & Gordon ME (1990) A musculoskeletal model of the human lower extremity: the effect of muscle, tendon and moment arm on the moment-angle relationship of musculotendon actuators at the hip, knee and ankle. J Biomech 23, 157-169.
Lakie M, Caplan N & Loram ID (2003). Human balancing of an inverted pendulum with a compliant linkage: neural control by anticipatory intermittent bias. J Physiol 551, 357-570.
Loram ID & Lakie M (2002a). Direct measurement of human ankle stiffness during quiet standing: the intrinsic mechanical stiffness is insufficient for stability. J Physiol 545, 1041-1053.
Loram ID & Lakie M (2002b). Human balancing of an inverted pendulum: position control by small, ballistic-like, throw and catch movements. J Physiol 540, 1111-1124.
Loram ID, Maganaris CN & Lakie M (2004). Paradoxical muscle movement in human standing. J Physiol 556, 683-689.
Morasso PG & Sanguineti V (2001). Ankle muscle stiffness alone cannot stabilize balance during quiet standing. J Neurophysiol 88, 2157-2162.
Smith JW (1957). The forces operating at the human ankle joint during standing. J Anat 91, 545-564.
Winter DA, Patla AE, Rietdyk S & Ishac MG (2001). Ankle muscle stiffness in the control of balance during quiet standing. J Neurophysiol 85, 2630-2633.
