Physiology News Magazine
Our shape is elastic, modular and held together by carbohydrate strings
Glycan strings tie collagen fibrils into shape-defining modules with intrinsic elasticity based on a springy sugar (Iduronate) and reversible intermolecular ratchets
Features
Our shape is elastic, modular and held together by carbohydrate strings
Glycan strings tie collagen fibrils into shape-defining modules with intrinsic elasticity based on a springy sugar (Iduronate) and reversible intermolecular ratchets
Features
John E Scott
Chemical Morphology, Manchester University, Manchester, UK
https://doi.org/10.36866/pn.56.22

Bodies are supramolecular organisations, but we are not molecular Lego-men. We respond elastically to internal and external stresses. This enables us to move and to recover from our encounters with the outside world. Much of this elasticity is proposed to reside in carbohydrate strings that tie us together.
Two views of connective tissues
‘Connective tissue is what you get rid of in order to work with cells’ (anonymous physiologist).
‘If by some magic solution one could dissolve all the connective tissues of the body, all that would remain would be a mass of slimy epithelium, quivering muscle and frustrated nerve cells’ (Arcadi (1952) quoting his unnamed teacher).
Complete agreement! But these two anonymous commentators are going in opposite directions. There is a third way. Connective tissue (CT) (skin, bones, tendon, cartilage, etc.) is shape. Shape is one of the most important issues in biology. Without a permanent shape central functions (digestion, circulation, etc.) could not have evolved. Ergo, CTs are of supreme importance. How do they do it? Is there a core concept?
Nearly 30 years ago I proposed a global definition, applicable to all CTs (or more precisely their extracellular matrices, ECMs) in all species at all stages in development as follows: ‘animal CTs are systems of insoluble fibrils and soluble polymers that evolved to take the stresses of movement and the maintenance of shape’ (Scott, 1975). The fibrils are proteins – collagen and elastin. They are ropes, resisting and transmitting tensile (pulling) stresses, visible as such from the very thin (~10nm) collagen fibrils in vitreous humour to the huge tendons that support large animals.
The soluble polymers are carbo-hydrates, anionic glycosaminoglycans (AGAGs, e.g. chondroitan, keratan and dermochondan sulphates). They are attached to proteins, thereby becoming proteoglycans (PGs). They resist compressive forces because they tend to swell in aqueous solutions, as all polymers do in good solvents, thus increasing their entropy. The many anionic groups (carboxylates, sulphate esters) they carry repel each other, expanding the polymers they are covalently attached to. Also important is the osmotic pressure of counterions (Na+, etc.) associated with the GAG anions. The total swelling pressure inflates the fibrillar matrix. This was elegantly proved by precipitating the polyanions with cobalt hexammine or cetylpyridinium. The volume occupied by the precipitated polyanion is much less than that in solution, effectively removing it from the tissue. PG-rich tissues (e.g. cartilage or corneal stroma) then shrink, losing their turgor (Hedbys, 1961) and elasticity.
The anatomy
How do the fibrils co-operate with the PGs? They cannot move independently or the shape of a CT would depend on its stressful history and a permanent shape would be impossible. The fibrils must be in a constant orientation. Collagen fibrils in, for example, tendons and corneal stroma, are in beautiful parallel arrays but conventional electron histochemistry shows no interfibrillar connections, although there must be structures which hold the fibrils in permanent orientations.
In fact, collagen fibrils are tied together by PG AGAG bridges. These are in solution in vivo and consequently invisible. They were discovered when electron-dense reagents (e.g. Cupromeronic Blue) were developed to stain AGAGs for electron microscopy (Scott, 1985). Cationic Cupromeronic Blue binds electrostatically to polyanionic AGAGs, precipitating them while preserving much of the shape, size and orientation of the AGAG. The dermochondan sulphate-containing PG was called decoran because it decorates the collagen fibrils in a beautiful regular pattern. Similar PGs containing keratan sulphate are found in the corneal stroma. The PG protein is attached non-covalently to the fibrils and AGAG chains aggregate head-to-tail across the interfibrillar gap (Fig. 1).
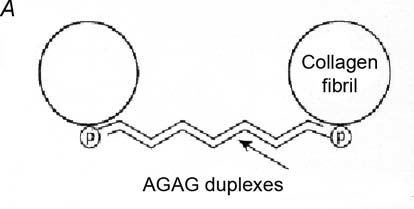
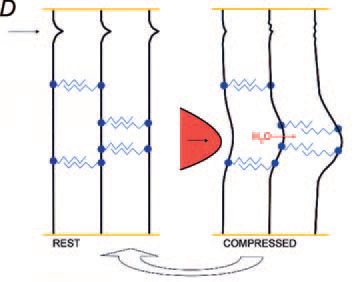
Not only are the PGs bound to collagen fibrils, thus stopping them from wandering under stress, but they bridge and tie the fibrils together in register. The lengths of the AGAG chains correlate with the interfibrillar separation, being short (e.g. about 15 kDa in tendon) where fibrils are densely packed, rising to 50 kDa in cornea, where fibrils of constant diameter are precisely well separated, and to 350 kDa in the very dilute vitreous humour. The bridge-fibril interaction repeats regularly along the fibril, i.e. modularly. I therefore termed it the ‘shape module’. ECM cells unable to make the PG protein were unable to form shape modules and the ‘ECM’ they produced was totally disordered.
Shape modules are found in all ECMs. They are ubiquitous and overwhelmingly dominant structures in animals as remote as holothurians. Thus, animal shapes are stabilised by carbohydrate strings, i.e. the AGAG chains in interfibrillar bridges.
The physiology
Although CT ECMs provide a robust shape, they must deform reversibly in response to internal and external stresses. 100% return to the original shape is mandatory, post stress. Clearly, 99.9% will not do. Shape module components must therefore be (or be part of) elastic structures.
Fibrillar elastic properties are well described. Elastin elongates reversibly (up to 200%), driven by hydrophobic and entropic interactions. It is ‘animal rubber’. Collagen fibrils are elastic to a much smaller degree, elongating reversibly by ~10%, probably by rearrangements of collagen molecules within the fibrils.
PG AGAG elasticity is relatively uncharted. Polymers entangle and disentangle, thus contracting and expanding, but there is little organised structure in this process and it is probably not significant in tissue elasticity.
Two new mechanisms were proposed (Scott, 2003), one depending on the spring-like behaviour of a specific sugar (L-iduronate) in the AGAG chain, and the other in which shape module AGAG bridges partially disaggregate under tensile stresses.
L-iduronate springs
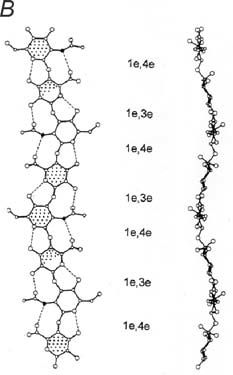
The AGAG sugars (D-galactose, D-galactosamine, D-glucosamine and D-glucuronate) except one, (L-iduronate), are stable 4C1 pyranose conformers (Fig. 2). L-iduronate occurs in three conformers (1C4, 4C1 and 2S0) with almost identical energies (Casu et al. 1988). One easily changes to another. Nevertheless, NMR shows that the 1C4 and 2S0 forms are preferred in aqueous solutions of dermochondan sulphate, which is characteristic of shape modules. Molecular modelling showed that 1C4 and 2S0 forms are more compact (‘thinner’) along the AGAG chain axis than the 4C1 form, and a chain of 1C4 forms could stretch by 7-10% under tensile stress to the 4C1 forms. On release the chain would shorten as L-iduronate contracted to the preferred compact forms (Fig. 2).
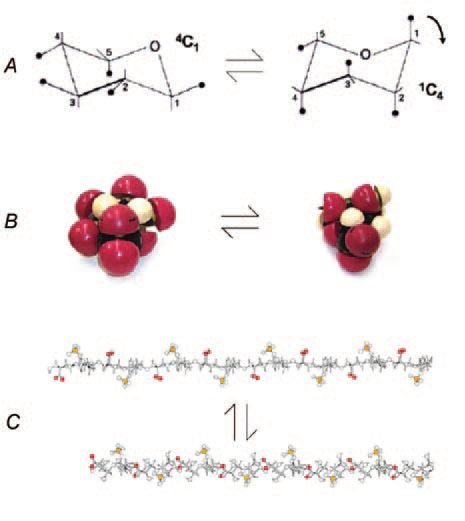
For the first time this schema provides a mechanical raison d’etre for L-iduronate, which is formed by epimerisation in the polymer of D-glucuronate. This energy-expensive bit of biosynthesis was formerly without a rationale. It suggests why the content of L-iduronate varies greatly in dermochondans from different tissues.
Flexible tissues such as skin are characterised by large scale epimerisation (> 90%), whereas in rigid structures such as cartilage conversion stops at a much lower (~10%) level. Turgor in cartilages is high, so that L-iduronate may normally be in the fully stretched form, perhaps adopting compact forms under compression. L-iduronate/D-glucuronate ratios in corneal stroma are higher than in cartilage, consistent with the idea that cornea requires rigidity to maintain shape and hence its optical properties, but also elasticity to respond rapidly and reversibly to stresses associated with blinking and eye movements.
Atomic levers may control elasticity in L-iduronate-containing AGAGs
Direct physical evidence indicates that tensile stress changes sugar conformations. Using atomic force microscopes, single polysaccharide molecules were stretched while observing the relationship between lengths and applied force. Conversions from chair to boat forms were engineered by so-called ‘atomic levers’ (Marszalek et al. 1999), axial glycosidic bonds which, when pulled, forced the pyranose ring into a conformation in which the stress is transmitted in a straight line along the polymer chain. It is deducible from the work of Marszalek and co-workers that chondroitan 4 & 6 sulphates, hyaluronan and keratan sulphates will not stretch under tensile stress since their glycosidic bonds are equatorial rather than axial, offering no leverage to force conformational changes. Their sugars are fully extended anyway. On the contrary, the compact 1C4 and 2S0 forms of L-iduronate have axial glycosidic links. These atomic levers enhance the effect of tensile stress on the change between conformers (Fig. 2), thus improving intrinsic elasticity. Heparan sulphate, which is not an ECM AGAG, nevertheless should show L-iduronate elasticity, relevant to its role in flexible cell membranes.
L-iduronate confers small scale elasticity, but there are requirements for larger scale reversible deformabilities. Corneal stroma swells reversibly 5-fold and the spring mechanism cannot accommodate that. An intermolecular mechanism was proposed to deal elastically with large scale deformations (Scott, 2003).
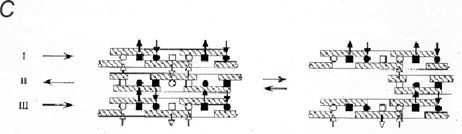
The PG sliding-filament mechanism of shape module elasticity
AGAG bridge structure in the shape module is based on non-covalent interactions. Electron microscopy, NMR and molecular modelling indicate that the AGAG chains are probably layered on top of each other, head-to tail, which allows hydrophobic and H-bonds to form between neighbouring AGAGs (Fig. 1). This stringent lock-and-key complementarity repeats along the polymer chains. In the case of hyaluronan the H-bonds were shown by rheo NMR to break reversibly under shear stress. Thus aggregated AGAGs can be pulled apart in a slippage process, during which the low energy hydrophobic and H-bonds are broken. Slippage occurs along the AGAG bridge axis, in the direction of applied tensile stress. On releasing the stress slippage reverses as bonds originally present are reformed into the optimal (lowest energy) structure (Fig. 1).
In this scheme compressive forces are converted into tensile forces distant from the compression, disseminated throughout the tissue. The scheme predicts that when slippage exceeds a certain limit the tissue will disintegrate, probably irreversibly. Indeed, when corneal stroma swells beyond about 5-fold, the process becomes irreversible.
Coda
The requirement for elasticity in blood vessel walls, for example, is well recognised. The properties of elastic proteins (resilins, etc.) have been documented throughout animal biology. However, they are only part of the story. Their partners in shape-defining connective tissue must also be elastic if the tissue as a whole is to be elastic. The spring and dashpot properties of carbohydrates are now seen to complement elastin, etc. in providing elastic linkages in modular ECM structures, playing a vital role in maintaining shape.
References
Arcadi JA (1952). Book Review of Connective Tissues (Transactions of the first conference of the Macy Foundation). Bull Johns Hopkins Hospital 90, 334-335.
Casu B, Petitou M, Provasoli M, & Sinay P (1988). Conformational flexibility: a new concept for explaining binding and biological properties of iduronic acid-containing glycosaminoglycans. Trends Biochem Sci 13, 221-225.
Hedbys BO (1961). The role of polysaccharides in corneal swelling. Exp Eye Res 1, 81-91.
Marszalek PE, Pang Y-P, Li H, Yazal JE, Oberhauser AF & Fernandez JM (1999). Atomic levers control pyranose ring conformations. Proc Natl Acad Sci USA 96, 7894-7898.
Scott JE (1975). Composition and structure of the pericellular environment: physiological function and chemical composition of pericellular proteoglycan (an evolutionary view). Phil Trans Roy Soc Lond B 271, 235-242.
Scott JE (1985). Proteoglycan histochemistry – a valuable tool for connective tissue biochemists. Coll Rel Res 5, 541-598.
Scott JE (2003). Elasticity in extracellular matrix ‘shape modules’ of tendon, cartilage etc. A sliding proteoglycan-filament model. J Physiol 553, 335-343.