
Physiology News Magazine
Vasopressin may limit its own secretion with the help of pituicytes
Vasopressin released from the pituitary is a critical controller of body water balance. Like many hormones, it seems to be able to inhibit its own release. Jean-Marc Mienville and colleagues discuss the possible underlying mechanisms
Features
Vasopressin may limit its own secretion with the help of pituicytes
Vasopressin released from the pituitary is a critical controller of body water balance. Like many hormones, it seems to be able to inhibit its own release. Jean-Marc Mienville and colleagues discuss the possible underlying mechanisms
Features
Lia Rosso, Brigitta Peteri-Brunbäck, & Jean-Marc Mienville
CNRS – UMR 6548, Laboratoire de Physiologie Cellulaire et Moléculaire Université de Nice-Sophia Antipolis, Nice, France
https://doi.org/10.36866/pn.55.30

To maintain homeostasis, organisms often resort to various mechanisms of negative feedback. This is particularly true of secretory processes, a classical example being the output of catecholamines, which is self-regulated through inhibition of tyrosine hydroxylase (the enzyme that catalyzes the first step of catecholamine synthesis) by each intermediate product of the synthetic chain.
Recently, we have obtained evidence in support of yet another type of negative feedback involving a crosstalk between the neuronal and glial components of the neurohypophysis (Rosso et al. 2004). This gland comprises primarily three functional entities: the axon terminals of the hypothalamic magnocellular neurones that synthesize and secrete the hormones vasopressin and oxytocin; pituicytes (the glial component); and blood vessels, which drain the hormones from the sites of release and allow them to reach their cellular targets through the general circulation. Here we describe how vasopressin may regulate its own output by acting upon pituicytes, the specialized astrocytes of the neurohypophysis.
Since the late 1950s, work from several laboratories has suggested that pituicytes might be involved in the regulation of hormone secretion from the neurohypophysis. This idea was generated by electron microscopy studies indicating profound morphological changes in pituicytes during physiological states that involve a high output of neurohypophysial hormones. Numerous converging data led Hatton (1988) to propose a model whereby activated pituicytes retract both from between secretory terminals, thereby increasing their excitability, and away from the basal lamina of the perivascular space, whose increased contact with terminals ought to facilitate hormone release into the blood. Both vasopressin and oxytocin are synthesized in the magnocellular nuclei of the hypothalamus; they are axonally transported to the neurohypophysis where they are released into the blood. Vasopressin (or anti-diuretic hormone) plays a crucial role, among others, in the hydromineral balance of the organism by favouring water reabsorption by the kidney. Its output is primarily regulated by the electrical activity of magnocellular neurones. During dehydration, for example, the firing of these neurones is increased via activation of peripheral, central and intrinsic osmoreceptors sensing a rise in plasma osmolarity (see Bourque et al. 1994 for review). At the neurohypophysial level, this increased activity propagates to vasopressinergic axon terminals whose secretory vesicles release their content into the blood.
Relevant to our findings, two particular features of this secretion are (1) the fact that it also occurs within the interstitial space of the neurohypophysis, and (2) that ATP is present in the vesicles (see Rosso et al. 2004 for details). As a consequence, pituicytes are likely to be exposed to both vasopressin and ATP every time secretion is activated.
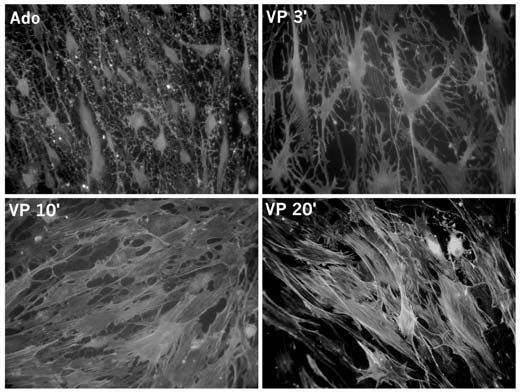
Because ATP is quickly broken down to adenosine by specific enzymes present in the neurohypophysis, we investigated whether the latter compound could be one of the signals involved in the morphological changes observed in pituicytes. Using in vitro cultures, we found that adenosine was indeed capable of dramatically modifying pituicyte shape from a flat and fusiform aspect to a shrunken cell body surrounded by long processes, a phenomenon called stellation (Rosso et al. 2002a). We are currently in the process of verifying whether this also occurs in vivo. Subsequently, we discovered that vasopressin was able to reverse stellation, bringing pituicytes back to their basal shape (Fig. 1; Rosso et al. 2002b). If ATP and vasopressin are coreleased within the pituicyte environment, what could be the net effect of two simultaneous signals having opposite actions? Our provisional hypothesis is that during the initial stages of secretion vasopressin receptors are internalized – and thus non-functional – due to the high concentrations of vasopressin ‘seen’ by the cells. This idea is borne out both by previous observations in various cell types and by our own unpublished data. Therefore, we believe that the purinergic effect (chemically both ATP and adenosine are purine compounds) initially prevails, and that adenosine-induced stellation in vitro corresponds to the morphological changes observed in vivo upon activation of the hypothalamo-neurohypophysial system. It should be noted that stellation reversal can be elicited by very low vasopressin concentrations (EC50 ~0.1 nM), so that following hormone clearance and/or adenosine breakdown, vasopressin might be able to negatively feedback on its own secretion by returning pituicytes to their resting morphology.
A second component of this putative negative feedback consists of the ability of vasopressin to release taurine from pituicytes. Taurine is known as an ubiquitous osmolyte in the regulation of cell volume, but its role in the neurohypophysis has more of a signalling nature, inasmuch as it inhibits vasopressin release by activating strychnine-sensitive glycine receptors located on neurohypophysial terminals (reviewed by Hussy, 2002). Here, the physiological significance is straightforward given the fact that hypotonic shocks increase taurine levels in the isolated neurohypophysis, which is consistent with the need to keep vasopressin levels low under these conditions. We, in turn, have demonstrated that vasopressin itself is capable of inducing pituicytes to release taurine, again suggesting a negative feedback mechanism for self-limitation of secretion (Rosso et al. 2004).
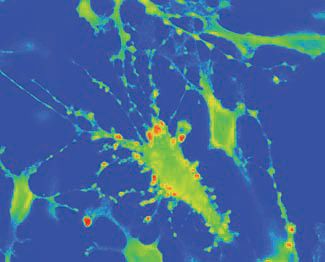
An intriguing aspect of vasopressin’s action on pituicytes was the formation of cellular protrusions intensely stained for taurine (Fig. 2). The nature and physiological role of these protrusions remain to be established. One important functional parameter of the taurine-releasing effect of vasopressin is that it is prevented in hypertonic conditions. Therefore, one can reason that during the initial stages of hormone demand, e.g. during dehydration, vasopressin will be unable to release taurine because of the hyperosmotic environment of the neurohypophysis, thereby allowing unimpeded output and full action of the hormone. Thereafter, when the water balance begins to be restored and the need for vasopressin subsides, a powerful negative feedback can be activated via taurine-induced blockade of release, which may help terminate the action of vasopressin and prevent water overload.
Most of these results were obtained in vitro. If they are confirmed in vivo, they will provide another example for the use of a negative feedback mechanism in the control of the organism’s secretions. Furthermore, such a mechanism would represent a novel physiological role for neuron/glia interactions.
References
Bourque CW, Oliet SH & Richard D (1994). Osmoreceptors, osmoreception, and osmoregulation. Front Neuroendocrinol 15, 231-274.
Hatton GI (1988). Pituicytes, glia and control of terminal secretion. J Exp Biol 139, 67-79.
Hussy N (2002). Glial cells in the hypothalamo-neurohypophysial system: key elements of the regulation of neuronal electrical and secretory activity. Prog Brain Res 139, 95-112.
Rosso L, Peteri-Brunbäck B, Vouret-Craviari V, Deroanne C, Troadec J-D, Thirion S, Van Obberghen-Schilling E & Mienville J-M (2002a). RhoA inhibition is a key step in pituicyte stellation induced by A1-type adenosine receptor activation. Glia 38, 351-362.
Rosso L, Peteri-Brunbäck B, Vouret-Craviari V, Deroanne C, Van Obberghen-Schilling E & Mienville J-M (2002b). Vasopressin and oxytocin reverse adenosine-induced pituicyte stellation via calcium-dependent activation of Cdc42. Eur J Neurosci 16, 2324-2332.
Rosso L, Peteri-Brunbäck B, Poujeol P, Hussy N & Mienville J-M (2004). Vasopressin-induced taurine efflux from rat pituicytes: a potential negative feedback for hormone secretion. J Physiol 554, 731-742.
