
Physiology News Magazine
The exciting mitochondrion
Evidence is emerging that mitochondria play an important role in modulating the excitability of muscle and other cells. Not bad for an organelle that may have started out as a bacterium ‘adopted’ by primordial cells
Features
The exciting mitochondrion
Evidence is emerging that mitochondria play an important role in modulating the excitability of muscle and other cells. Not bad for an organelle that may have started out as a bacterium ‘adopted’ by primordial cells
Features
D George Stephenson & *Niels Ørtenblad
The Muscle Research Group, Department of Zoology, La Trobe University, Melbourne, Victoria, Australia
*Institute of Sports Science and Clinical Biomechanics University of Southern Denmark, Odense, Denmark
https://doi.org/10.36866/pn.54.14

The mitochondrion has been long known as the powerhouse of the eukaryotic cell supplying most of the necessary ATP to keep it alive (Lehninger, 1965). More recent studies have shown that the mitochondrion also plays prominent roles in the regulation of heat production (Palou et al. 1998), formation of oxygen radicals (Duranteau et al. 1998), calcium signalling (Pozzan et al. 2000), glucose homeostasis (Maechler & Wohlheim, 2000), oxygen sensing (Duchen, 2000; Chandel & Schumacker, 2000) and apoptosis (Newmeyer & Ferguson-Miller, 2003).
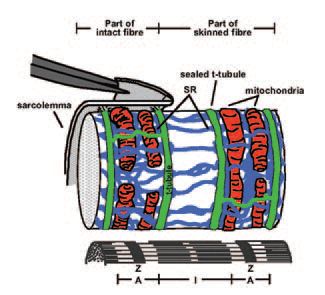
A recent study from our laboratory (Ørtenblad & Stephenson, 2003) has shown the mitochondrion may also regulate the excitability of the skeletal muscle fibre by a mechanism that is not dependent on changes in the intracellular levels of ATP, pH, Mg2+ or Ca2+. In this study, the ATP-producing ability of mitochondria was impaired with three molecularly diverse and commonly used mitochondrial inhibitors (azide, oligomycin and FCCP). When these mitochondrial antagonists were applied to a fully excitable ‘inside out’ rat muscle fibre preparation, where the composition of the myoplasmic environment was controlled with respect to [ATP], [Ca2+],[H+], [Mg2+] and other diffusible substances, the excitability of the preparation decreased in a dose-dependent and reversible fashion. The ‘inside out’ excitable muscle fibre preparation was developed in our laboratory based on the ‘mechanically skinned’ fibre preparation first introduced by Natori in 1954 (Natori, 1972), in which the sarcolemma is removed by microdissection under oil, leaving the cellular structure of the muscle fibre otherwise intact. In this preparation the transverse tubular (t-) system, characteristic of skeletal (and cardiac) muscle fibres, pinches off and seals off as the surface membrane is removed (Lamb et al. 1995; Launikonis & Stephenson, 2001). The t-system forms the major interface between the cell and its extracellular environment and, when sealed, it repolarises if the preparation is placed in a solution mimicking the myoplasmic environment (Lamb & Stephenson, 1990; 1994). This is because the Na+-K+ pumps located in the t-system re-establish the [Na+] and [K+] gradients across the sealed t-system membranes. The t-system can then be depolarised either by ion substitution or by electrical stimulation triggering propagated action potentials (Posterino et al. 2000). The t-system depolarisation induces Ca2+ release from the sarcoplasmic reticulum (SR) subsequently activates the contractile apparatus just as it does in an intact muscle fibre. A cartoon of this ‘inside out’ muscle fibre preparation displaying the relative position of mitochondria with respect to the t-system, SR and the myofibrillar component in a mammalian skeletal muscle fibre is shown in Fig. 1. In Fig. 2 are presented typical action-potential induced twitch responses in one such ‘inside out’ rat muscle fibre preparation in the presence and the absence of azide. Several lines of evidence indicate that the decrease in the size of the twitch response in the presence of the three mitochondrial ATP-producion inhibitors was caused by reduced fibre excitability due to depolarisation of the t-system (see Ørtenblad & Stephenson, 2003).
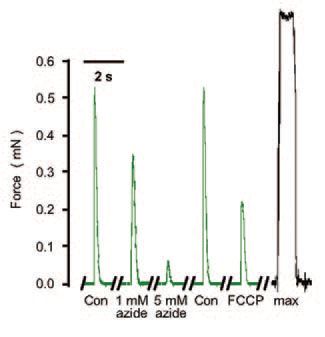
This phenomenon, in which impairment of mitochondrial ATP-producing function leads to graded depolarisation of the plasma membrane, reduced excitability and consequently reduced ATP consumption, is physiologically important because it is likely to play a key role in protecting the cellular ATP pool and thus safeguarding the cell from irreversible ATP-depletion induced injury. There are many observations of acute plasma membrane depolarisation during metabolic stress in several other cell types including cardiac fibres (Hasin & Barry, 1984), neurons (Buckler & Vaughan-Jones, 1998) glial cells (Brismar & Collins, 1993) and endothelial cells (Park et al. 2002). It is highly probable that a mechanism similar to that uncovered with the ‘inside out’ skeletal muscle fibre preparation is at work in most cells, providing the feed-back loop to maintain the balance between cell capacity for ATP production and ATP utilization.
The mechanism by which the inhibition of mitochondrial ATP-producing function causes depolarisation of the plasma membrane in the t-system is not known. It is probable that a chemical messenger is involved in the communication between the mitochondria and the t-system because there is no continuity or tight physical coupling between mitochondria and the t-system to facilitate a more direct type of interaction. Also, in accord with this proposition, toad muscle fibre preparations, where mitochondria are not located as closely to the t-system as in mammalian muscle fibres, were found to be less sensitive to the mitochondrial inhibitors tested than the rat fibres.
Previous observations on intact cardiomyocytes (Duranteau et al. 1998) showed that azide reversibly increased the production of reactive oxygen species (ROS) by the mitochondria and in parallel depressed myocyte contractility, as in the study on ‘inside-out’ rat skeletal fibres (Fig. 2). Hence, the mechanism by which impairment of ATP-producing capacity of mitochondria causes depolarisation of the plasma membrane may involve ROS production in the mitochondria. ROS would diffuse out of the mitochondria and act on membrane channels increasing the relative membrane permeability to Na+ compared to that of K+ as suggested in our experiments (Ørtenblad & Stephenson, 2003). A very similar process of mitochondria-controlled plasma membrane excitability to that described by us has been very recently found to operate in brain stem motoneurons (Bergmann & Keller, 2003). Also, Isaeva & Shirokova (2003) have recently shown that application of various mitochondrial inhibitors led to the loss of mitochondrial Ca2+ and promoted spontaneous Ca2+ release from the SR. It is then possible that ROS generated in the mitochondria during metabolic stress would also act on the SR Ca2+-release channels. A cross-talk between mitochondria and the sarcoplasmic reticulum would further support the idea that mitochondria play a much more active role than previously thought in modulating excitation-contraction coupling in skeletal muscle fibres.
Here it has been argued that ROS may play a critical role in facilitating the cross-talk between mitochondria and the t-system and between mitochondria and SR. However, one cannot exclude the possibility that other factors produced in the mitochondrion when its ATP-production function is impaired act as messengers between the mitochondria and other cellular membranes because there is clear evidence that many chemical messengers produced in the mitochondria can modulate cellular activity (Duchen, 2000).
Whatever the precise pathway of mitochondrial signalling to the plasma membrane may be, the finding that the mitochondrion could play a role in determining the excitability of the muscle cells should further transform this organelle into a fascinating object of study by excitable tissue physiologists.
References
Bergmann F & Keller BU (2003). Impact of mitochondrial inhibition on excitability and cytosolic Ca2+ levels in brain stem motoneurons from mouse. J Physiol DOI: 10.1113/053900.
Brismar T & Collins VP (1993). Effects of external cation concentration and metabolic inhibitors on membrane potential of human glial cells. J Physiol 460, 365-383.
Buckler KJ & Vaughan-Jones RD (1998). Effects of mitochondrial uncouplers on intracellular calcium, pH and membrane potential in rat carotid body type I cells. J Physiol 513, 819-833.
Chandel NS & Schumacker PT (2000). Cellular oxygen sensing by mitochondria: old questions, new insight. J Appl Physiol 88, 1880-1889.
Duchen, MR (2000). Mitochondria and calcium: from cell signalling to cell death. J Physiol 529, 57-68.
Duranteau J, Chandel NS, Kulisz A, Shao Z & Schumacker PT (1998). Intracellular signaling by reactive oxygen species during hypoxia in cardiomyocytes. J Biol Chem 273, 11619-11624.
Hasin Y & Barry, WH (1984). Myocardial metabolic inhibition and membrane potential, contraction, and potassium uptake. Am J Physiol 247, H322-H329 .
Isaeva EV & Shirokova N (2003). Metabolic regulation of Ca2+ release in permeabilized mammalian skeletal msucle fibres. J Physiol 547, 453-462.
Lamb GD & Stephenson DG (1990). Calcium release in skinned muscle fibres of the toad by transverse tubule depolarization or by direct stimulation. J Physiol 423, 495-517.
Lamb GD & Stephenson DG (1994). Effects of intracellular pH and [Mg2+] on excitation-contraction coupling in skeletal muscle fibres of the rat. J Physiol 478, 331-339.
Lamb GD, Junankar PR & Stephenson DG (1995). Raised intracellular [Ca2+] abolishes excitation-contraction coupling in skeletal muscle fibres of rat and toad. J Physiol 489, 349-362.
Launikonis BS & Stephenson DG (2001). Effects of membrane cholesterol manipulation on excitation-contraction coupling in skeletal muscle of the toad. J Physiol 534, 71-85.
Lehninger AL (1965). The mitochondrion: molecular basis of structure and function. 2nd print. New York: WA Benjamin.
Maechler P & Wollheim CB (2000). Mitochondrial signals in glucose-stimulated insulin secretion in the beta cell. J Physiol 529, 49-56.
Natori R (1972). Some physiological aspects of internal membrane of skeletal muscle fibres. Jikeikai Med J 19, 51-64.
Newmeyer DD & Ferguson-Miller S (2003). Mitochondria: releasing power for life and unleashing the machineries of death. Cell 112, 481-490.
Ørtenblad N & Stephenson DG (2003). A novel signalling pathway originating in mitochondria modulates rat skeletal muscle membrane excitability. J Physiol 548, 139-145.
Palou A, Pico C, Bonet ML & Oliver P (1998). The uncoupling protein, thermogenin. Int J Biochem Cell Biol 30, 7-11.
Park KS, Jo I, Pak K, Bae SW, Rhim H, Suh SH, Park J, Zhu H, So I & Kim KW (2002). FCCP depolarizes plasma membrane potential by activating proton and Na+ currents in bovine aortic endothelial cells. Pflügers Arch 443, 344-352.
Posterino GS, Lamb GD & Stephenson DG (2000). Twitch and tetanic force responses and longitudinal propagation of action potentials in skinned skeletal muscle fibres of the rat. J Physiol 527, 131-137.
Pozzan T, Magalhaes P & Rizzuto R (2000). The comeback of mitochondria to calcium signalling. Cell Calcium 28, 279-283.
