
Physiology News Magazine
Limbs in limbo: the problem of targeting
Keri Lee Page focuses on the problems inherent in an action as apparently simple as scratching an itch
Features
Limbs in limbo: the problem of targeting
Keri Lee Page focuses on the problems inherent in an action as apparently simple as scratching an itch
Features
Keri Lee Page
Department of Zoology, University of Cambridge
https://doi.org/10.36866/pn.50.12

If you had some food on your cheek, you’d probably wipe it away and if you had an itch you’d most likely scratch it. We do these things instinctively, without a second thought. But directing the trajectory of a moving limb so that it meets up with a target in space is a complex computational task. For targeting to be successful, a human or animal must translate a set of spatial co-ordinates – the target position, encoded by exteroceptive sensory inputs – into a coordinated temporal activation of motor neurones, which is ultimately responsible for the final movement. There is also the problem of kinematic redundancy. This is when the same target can be reached by an infinite number of different motor patterns, requiring that targeting networks must have some criteria for ‘selecting’ the most appropriate motor action. Nevertheless most animals, from cockroaches to cats and cephalopod molluscs, can do it all as well as we can (Figure 1).
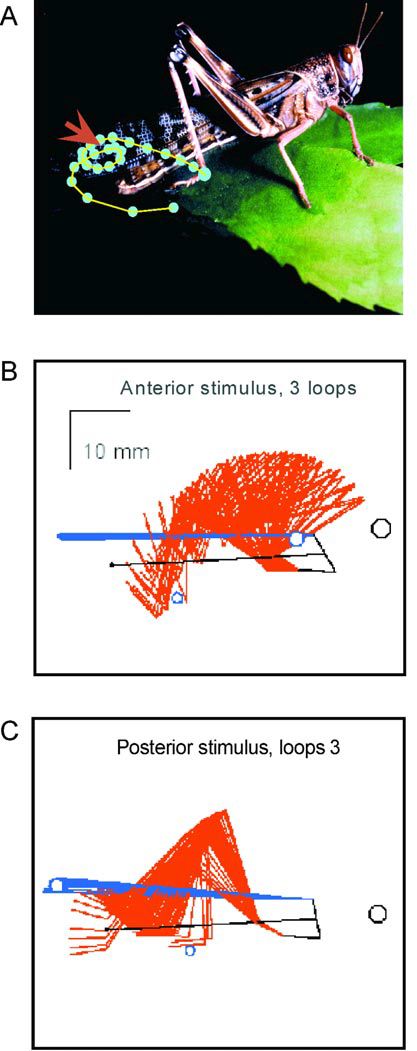
Much of what we currently know about vertebrate limb targeting comes from studies of episodic reaching in primates and rhythmic scratching in cats. In the early 1900s Sherrington demonstrated that chemical or tactile stimulation of the body surface in cats and dogs can elicit rhythmic directed scratching movements of a hindleg. Many animals have scratch reflexes that have several forms, depending on the location of the stimulus site. This is well known in the frog and turtle but is not the case with cats. In turtles three distinct reflexes exist (rostral, caudal and pocket scratches) with blends of each occurring at stimulus transition sites.
Invertebrates do remarkably well at the same tasks. Locusts clean their antennae with an antennal cleaning groove on the tarsus. This groove is brought into contact with an antenna by a stereotyped three-step motor sequence involving both the head and the forelegs (O’Shea, 1970). Targeting can also play a role in other contexts: in stick insects, for example walking and climbing use targeting. The middle and hind legs are targeted to step down directly behind the tarsus of the ante- rior leg, no matter how the position of this limb is perturbed (Cruse, 1979).
Targeted scratching in locusts
My research in Dr T Matheson’s lab in the University of Cambridge Zoology Department focuses on targeted scratching in locusts as a model system, and addresses the problems inherent in an action as apparently simple as scratching an itch on a wing (Matheson, 1997).
Tactile stimulation along the length of the wings elicits different targeting movements of the hindleg that scratch the stimulus site (Figure 1). For this targeting to be successful, the stimulus position first has to be encoded. This is usually done by way of a somatotopic map of afferent neurones from the mechanosensory hairs on the body surface. These arborisations synapse onto interneurones at the second level of the map in such a way that each interneurone represents a distinct area of the body surface called a receptive field.
Maps have been described for the mechanoreceptors on locust legs, but have not yet been described for the wing surface. As a first step, a count and distribution analysis of the wing hairs has been conducted. This reveals a characteristic spread of hairs along the main veins of the forewing. Where hairs are more densely distributed, the likelihood of eliciting a scratch with tactile stimulation is increased, resulting in probability differentials across the wing surface (Figure 2).
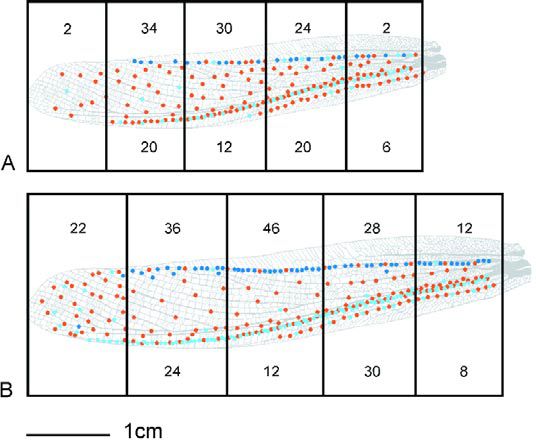
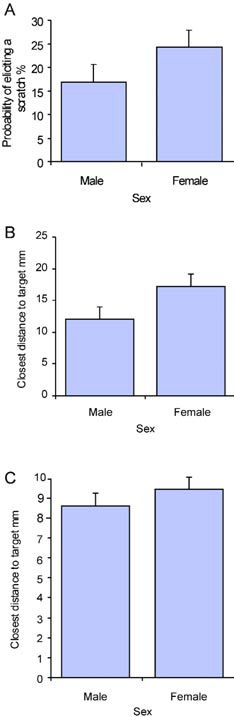
Surprisingly, there is a significant difference in the number of hairs between males and females – over and above that which would be expected due to the females’ larger body size. The larger number of hairs in females correlates with an overall increased probability of scratching for a given stimulus. Despite the larger number of mechanoreceptors on female wings, the females do not target with greater accuracy than males (Figure 3). This may indicate that increased sensory input causes greater levels of excitation in the central networks of females but that receptive fields are no more finely tuned by the presence of additional hairs. In other words, the hairs may synapse onto the same number of interneurones in both sexes.
The position of a limb is known by a ‘position sense’ arising mainly from proprioceptors within the joints. In locust scratching, hind leg position is primarily described by feedback from the femoral chordotonal organ. This receptor spans the femur-tibia joint and monitors the static position of the limb as well as direction, velocity and acceleration of any tibial movement (Figure 4).
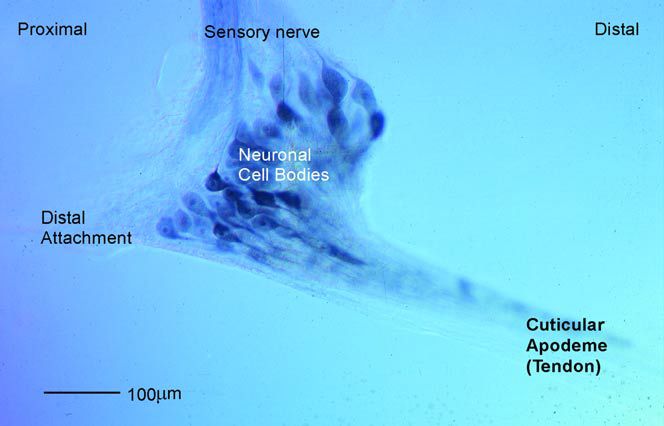
As the tibia is moved, the cuticular apodeme (tendon) stretches the body of the chordotonal organ accordingly, resulting in stimulation of the receptors. The receptors project into the central nervous system where their patterns of branching correlate with each response type. Identified local and ascending, intersegmental interneurones have been proposed as candidate neurones for the convergence of spatial information from the wing and positional information from the chordotonal organ of the hind leg. Interneurones with bilateral arborisations may be responsible for the distribution of spatial information that enables simultaneous targeting of the contralateral leg. Limbs in other body segments may also receive information from ascending interneurones, enabling interganglionic co-ordination (Matheson, 2002). This produces reflexes in the middle and forelegs which contribute to increased body stability while the hind leg is busy scratching.
Interneurones involved in collation must be integral to the network responsible for computing the final motor pattern. Non-spiking interneurones in the metathoraic ganglion which are known to control sets of leg motor neurones, are likely to be responsible for much of the interjoint coordination that is characteristic of a scratch.
Are central pattern generators involved in targeted scratching?
In locusts, scratches are incredibly variable. This suggests two possible scenarios: firstly there may be many distinct receptive fields that elicit discrete motor patterns which differ along the anterior to posterior axis. Alternatively, there may be a single motor pattern that is modulated in a continuously variable way by somatosensory feedback from the wing hairs. Recent work in the lab suggests that the latter explanation is correct. Descriptions of the circuitry that might underlie these alternatives, and the evidence that supports them is convoluted.
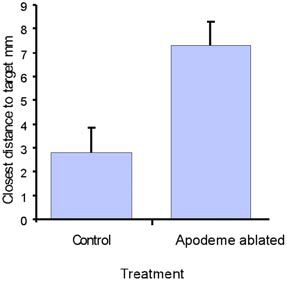
The existence of central pattern generators (CPGs) is supported heavily by studies of vertebrate targeting. The scratch reflex can be elicited in the decerebrate cat and spinal turtle (Robertson et al 1985). Some studies on locust scratching indicate discrete motor patterns for different stimulus sites. This too suggests the existence of a set of distinct CPGs, being activated by specific inputs. These distinct patterns remain in a de-efferented and decerebrate animal (Berkowitz et al 1996). My work, however, indicates that interfering with proprioceptive feedback has a significant effect on the motor pattern’s efficacy (Figure 5). Such findings would imply that feed- back is used to fine-tune the outputs from CPGs. Moreover, other work in the lab suggests that for sites on a wing, a single CPG output exists, that is strongly modulated by somatotopic input encoding the stimulus site. An alternative view of motor pattern generation is therefore being considered at present: is a rhythmic CPG in the traditional sense actually present at all? An alternative is that a population- coding scheme amongst interneuones may produce both linear targeting and under some conditions, cyclical repeated movements. Such a notion might describe the generation of cyclic targeting movements without relying on the standard concept of an autonomous oscillating unit.
In order for targeting to remain accurate, the networks must behave plastically with regard to external perturbations. For example, if a load is added to the hindleg of a scratching locust, feed- back from the campaniform sensilla and femoral chordotonal organ allows compensation for the extra weight, so that accuracy is not reduced. In frogs too, loading does not affect the animal’s ability to remove the stimulus. It will be interesting to see from our future experiments with locusts, how manipulating the proprioceptive signals from the chordotonal organ will affect targeting accuracy and whether the system is plastic enough to recalibrate the proprioceptive feedback over time, bringing the accuracy back into line.
Future implications of targeting studies
Targeting studies have wide ranging implications for technology and future research. The principal focus of vertebrate studies is to consider mapping in the spinal cord and also the role the motor cortex plays in the processes linking sensation and action. Mathematical models of these processes may eventually give rise to developments in robotic prosthetic-limb design. Invertebrate studies too, have important implications for robotics, since the invertebrate targeting paradigm should prove easier to model. Modelling of neural networks involved in the targeted stepping movements of stick insects has led to the development of WalkNet – an artificial neural network that controls six-legged walking (Cruse et al 1998). WalkNet has since been proven successful by its implementation in Tarry, a six-legged robotic insect (Figure 6) Biomimetic robots like these not only have consequences for the future of autonomous robots but also for the study of targeting, as the robots themselves throw up new lines of enquiry in the real animals studied by neuroscientists.


REFERENCES
BERKOWITZ A & LAURENT G (1996). Central generation of grooming motor patterns and interlimb coordination in locusts. J Neurosci. 16, 8079-8091.
CRUSE H (1979). The control of the anterior extreme position of the hindleg of a walking insect, Carausius morosus. Physiol Entomol 4, 121-124..
CRUSE H, KINDERMANN T, SCHUMM M, DEAN J & SCHMITZ, J (1998). Walknet – a biologically inspired network to control six-legged walking. Neural Netw 11, 1435-1447.
MATHESON T (1997). Hindleg targeting during scratching in the locust. J Exp Biol 200, 93-100.
MATHESON T (2002). Metathoracic neurones integrating intersegmental information in the locust. J Comp neurol 114, 95-114.
O’SHEA MR (1970). The antennal cleaning reflex in the desert locust schistocerca gregaria (Forsk.). In Proc Int Study Conf Current & Future Problems of Acridology. London. Pp 55-59.
ROBERTSON GA, MORTIN LI, KEIFER J & STEIN PSG (1985). J Neurophysiol 53, 1517-1534.
