
Physiology News Magazine
Nitric oxide microsensors
Roger Wadsworth reports on a symposium to discuss the physiological measurement of nitric oxide using electrochemical microsensors held at the European Society for Microcirculation 2002 meeting in Exeter
Features
Nitric oxide microsensors
Roger Wadsworth reports on a symposium to discuss the physiological measurement of nitric oxide using electrochemical microsensors held at the European Society for Microcirculation 2002 meeting in Exeter
Features
Roger Wadsworth
Department of Physiology & Pharmacology University of Strathclyde
https://doi.org/10.36866/pn.50.19

There is impressive evidence that derangements in nitric oxide (NO) occur in all of the main cardio- vascular diseases. This has fuelled continuing interest and speculation in the possibility that alteration in NO may be causative in certain cardio- vascular diseases, or that delivery of additional NO may be beneficial in therapy. This evidence, though extensive, is almost all indirect, being based principally on interpretation of end- organ responses to inhibitors of NO synthase, and the detection of by-products of NO metabolism. The development of the NO microsensor provides a method for real-time measurement of NO concentration, and a symposium was held at the European Society for Microcirculation 2002 meeting in Exeter (August 2002), to discuss the Physiological measurement of NO using electrochemical microsensors.
At the symposium, Malinski described the initial concept of the NO microsensor and its subsequent development into a variety of configurations suitable for particular applications (Malinski & Taha, 1992). Devynck described the advantages and limitations of coated microelectrodes to monitor NO from cultured vascular cells (Pontié et al 2000). Mas described the application of a NO microsensor to measurement of NO in vivo, using the corpus cavernosum penis as a model vascular bed (Escrig et al 1999). Kutzsche et al (1999) described the effects of hypoxia and reoxygenation on cerebral NO concentrations in pigs. Delli Gatti described the effect of plasma lipoproteins on NO production in cultured endothelial cells (Vergnani et al 2000). Stankevicius et al (2002) used NO microsensors to show that nitric oxide release in arteries from renal hypertensive rats is impaired.
There was also a workshop oriented to practical applications and technical questions, which generated a lively and spontaneous discussion. Boer outlined many pitfalls but ultimate success with the measurement of NO measurements in isolated arteries (Piepot et al 2000). Gonzalez-Mora described equipment for differential pulse voltammmetry (Escrig et al 1999). Zhang et al (2000) described that characteristics of a commercially available series of NO microsensors.
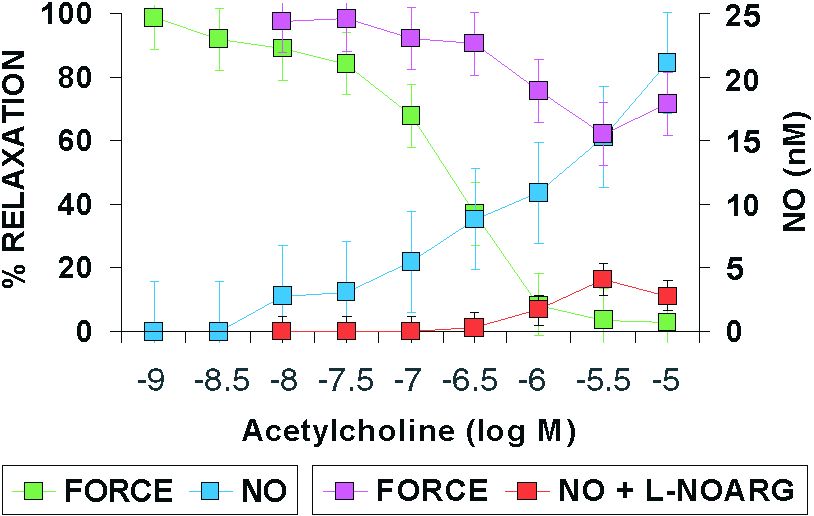
One example of the application of NO microsensors in blood vessels is shown in Figure 1. A ring of rat mesenteric artery was held in a small vessel myograph in Krebs solution. A NO microsensor (30 micrometres diameter) was inserted into the lumen using a micromanipulator. Relaxation of the artery with acetylcholine was accompanied by NO formation, detected at the microsensor. The concentration of NO detected was approximately 10 nM at maximal relaxation; however, the endothelium has a considerable reserve capacity and higher concentrations of acetylcholine generated more NO, over 20 nM. The NO synthase inhibitor L-nitro-L-arginine 100 µM caused a very pronounced, though not complete, block of NO formation and relaxation.
Anyone interested in the technique or applications is welcome to contact R M Wadsworth for further information (r.m.wadsworth@strath.ac.uk).
Our present state of knowledge of the role of NO in cardiovascular disease can be likened to someone who has heard of, but never seen, an elephant – they know approximately what it looks like and where it can be found, but they have little knowledge of its behaviour, its function, or how to make more of it (Figure 2).

Acknowledgement
The symposium was supported by educational grants from the British Heart Foundation, the Wellcome Trust, World Precision Instruments Inc, Servier Young Investigator Awards, The Physiological Society and Pfizer Ltd.
References
Escrig A, Gonzalez-Mora JL, Mas M (1999) Nitric oxide release in penile corpora cavernosa in a rat model of erection J Physiol 516 261-269.
Kutzsche S, Kirkeby OJ, Rise IR, Saugstad OD (1999) Effects of hypoxia and reoxygenation with 21% and 100% oxygen on cerebral nitric oxide concentration and microcirculation in newborn piglets. Biol Neonate 76, 153-167.
Malinski T, Taha Z (1992). Nitric oxide release from a single cell measured in situ by a porphyrinic-based microsensor. Nature 358, 676-678.
Piepot HA, Boer C, Groeneveld ABJ, van Lambalgen AA, Sipkema P (2000) Lipopolysaccharide impairs endothelial nitric oxide synthesis in rat renal arteries. Kidney Int 57, 2502-2510.
Pontié M, Gobin C, Pauporté T, Bedioui F, Devynck J (2000) Electrochemical nitric oxide microsensors: sensitivity and selectivity characterization. Anal Chem Acta 411, 175-185.
Simonsen U, Wadsworth RM, Buus NH, Mulvany MJ (1999) In vitro simultaneous measurements of relaxation and nitric oxide concentration in rat superior mesenteric artery. J Physiol 516, 271-282.
Stankevicius E, Martinez AC, Mulvany MJ, Simonsen U (2002) Blunted acetylcholine relaxation and nitric release in arteries from renal hypertensive rats. J Hyperten 20, 1571-1579.
Vergnani L, Hatrik S, Ricci F, Passaro A, Manzoli N, Zuliani G, Brovkovych V, Fellin R, Malinski T (2000) Effect of native and oxidised low-density lipoprotein on endothelial nitric oxide and superoxide production. Circulation 101, 1261-1266.
Zhang X, Cardosa L, Broderick M, Fein H, Lin J (2000) An integrated nitric oxide sensor based on carbon fiber electrode coated with selective membranes. Electroanalysis 12, 1113-1117. Adapted from an article published in the British Society for Cardiovascular Research Bulletin
