
Physiology News Magazine
MOLECULAR DETERMINANTS OF THE ‘M-CURRENT’
Here, Alex Selyanko explores the M-current and gives evidence that the sub-unit composition of M-channels may vary during development.
Features
MOLECULAR DETERMINANTS OF THE ‘M-CURRENT’
Here, Alex Selyanko explores the M-current and gives evidence that the sub-unit composition of M-channels may vary during development.
Features
Alex Selyanko
Department of Pharmacology, University College London, London WC1E 6BT
https://doi.org/10.36866/pn.43.9
Twenty years ago Brown and Adams discovered a new mechanism for spike adaptation in neurones – a potassium current which slowly activates on membrane depolarisation and prevents repetitive discharges (Brown & Adams, 1980). The Mcurrent 1s expressed in many autonomic and central neurones where its slow activation is responsible for their ‘phasic’ behaviour (Figure 1 A). Inhibition of the M-current by muscarinic receptor stimulants (hence the name ‘M-current’) or a specific blocker, XE991, causes the neurones to fire continuously (Figure 1B).
Molecular identification
The M-current was identified after two other major voltage-dependent K+ currents, the delayed rectifier and the A-current, and its channels were molecularly defined only two years ago. In 1998 Wang et al showed that the M-channels can be produced by heteromeric assembly of KCNQ2 and KCNQ3. These two subunits are expressed in brain and autonomic ganglia and mutations in either of them produce a benign form of epilepsy in newborn humans (Biervert et al, 1998; Charlier et al, 1998; Singh et al, 1998; Lerche et al, 1999). KCNQ2 and KCNQ3 are encoded by genes related to KCNQ 1 (Yang et al, 1997) and KCNQ4 (Kubisch et al, 1999), mutations in which produce cardiac long QT syndrome and deafness respectively, and KCNQ5 (Lerche et al, 2000; Schroeder et al, 2000), whose possible link to inherited diseases is still to be determined.
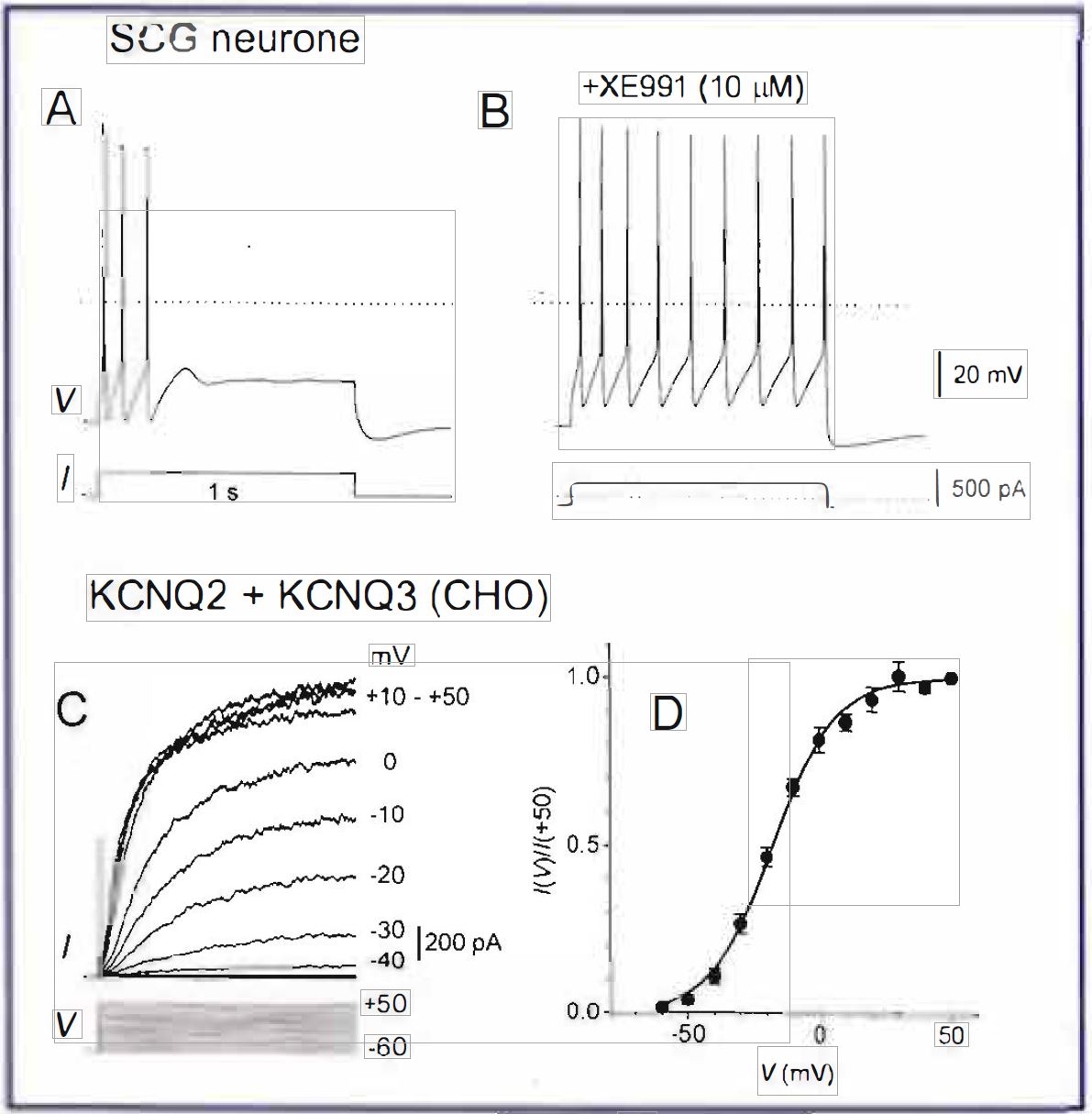
KCNQ2+KCNQ3 channels expressed in mammalian cells show a slow voltage-dependent activation, with a delay of tens of milliseconds even at very positive potentials (Figure 1C). This is consistent with the time course of spike adaptation in neurones (compare with Figure 1 A). The threshold of activation of KCNQ2+KCNQ3 channels is negative (close to ~-60 mY – the resting membrane potential in many types of neurone; Figure 1D), in accordance with the membrane depolarisation usually observed on muscarinic inhibition of the M-current.
Taken together, genetic evidence for the role of KCNQ2 and KCNQ3 channels in limiting neuronal excitability, their overlapping distribution in the nervous system as well as the pharmacological and biophysical similarities between the M-channels and heterologously expressed KCNQ2+ KCNQ3 channels, strongly suggest that KCNQ2/3 heteromers are the major contributors to the M-current (Wang et al, 1998). Functional parameters of M-type KCNQ2/3 channels are also adjusted by expression of different splice variants of KCNQ2 (those with a longer C-tail make the M-current slower; Pan et al, 2001) and an accessory subunit of the HERG potassium channel in the heart, KCNE2 (which makes the M-current faster; Tine] et al, 2000).
However, there is now accumulating evidence that within the neurone, and at some stage of its development, the subunit composition of Mchannels may be different, or may even not be limited to just KCNQ2 and KCNQ3 subunits. First, only KCNQ2 were detected in terminal fields in the human cortex and hippocampus suggesting that homomeric KCNQ2 channels may play a specific role in regulation of action potential propagation and neurotransmitter release (Cooper et al, 2000). (Interestingly, an M channel blocker linopirdine is a cognition enhancer which augments depolarizationinduced transmitter release in the cortex and which is under consideration for potential treatment of Alzheimer’s disease.) Second, during early postnatal development there is a delay in expression of KCNQ3, raising the possibility that at this stage homomeric KCNQ2 may contribute to the M-current (Tinel et al, 1998). Third, KCNQ5 is also expressed in the nervous system where it overlaps with KCNQ2 and KCNQ3 (Schroeder et al, 2000). Since KCNQ5 can form heteromers with KCNQ3 and when expressed heterologously, either as a homo or heteromer, is capable of generating M-type currents, it is feasible that it may also contribute to the M-current.
Structure and regulation
KCNQl-5 have between 679 (KCNQl) and 932 (KCNQ5) amino acids. Like other voltagedependent potassium channels they have N and C termini (both intracellular), six transmembrane domains with a voltage sensor in the fourth one, and a pore-forming P-loop. A subunit assembly domain is located at the mid-point of the C terminus, and both C and N termini contain many potential regulatory sites. Although KCNQ 1-5 have only 40-65% overall identity, restricted mainly to the transmembrane domains, the Ploop and the assembly domain, they all generate M-type currents with characteristic voltagedependence and kinetics, and all can be inhibited via muscarinic MI receptors, thus satisfying the criteria for M-channels (Selyanko et al, 2000). Nevertheless, KCNQ 1-5 are not identical in their responses to modulation. In view of the differential distribution of KCNQ I, KCNQ4 and KCNQ2,3&5 channels, and their involvement in different functions, selective modulation of individual subunits may give ultimate control over these functions.
Just a few examples. KCNQ2/KCNQ3 currents can be increased by intracellular cyclic AMP, through a phosphorylation site in the KCNQ2 amino terminus (Schroeder et al, I 998). A new anticonvulsant retigabine selectively activates ‘neuronal’ (KCNQ2-5) M-channels by shifting their activation curves to the left, without affecting the ‘cardiac’ (KCNQl) ones (Schroeder et al, 2001). A specific blocker of M-channels XE99 l is several times more potent against KCNQ2-5 than KCNQ I channels produced by co-expression with KCNE 1 (Wang et al, 2000). These differences are important because even a small change in the M-current may have a strong functional effect: mutations in KCNQ2/KCNQ3 leading to a form of epilepsy reduce the Mcurrent only by 25-30% (Schroeder et al, 1998). The structural determinants of M-channel inhibition/activation by various modulators, including neurotransmitters, is a major challenge for future investigation.
References
Biervert C et al (1998) Science 279: 403-406.
Brown DA & Adams PR (1980) Nature 283: 673-676.
Charlier C et al (1998) Nature Genetics 18: 53-55.
Cooper EC et al (2000) Proc Nat Acad Sci USA 97: 4914-4919.
Kubisch C et al (1999) Cell 96: 437-446.
Lerche H et al (1999) Ann Neurology 46: 305-312.
Lerche C et al (2000). J Biol Chem 275: 22395-22400
Pan Z et al (2001). J Physiol 531: 347-358.
Schroeder BC et al (1998) Nature 396: 687-690.
Schroeder BC et al (2000). J Biol Chem 275: 24089- 24095.
Schroeder RL et al (2001) Biophys J 80: 214a.
Selyanko AA et al (2000). J Physiol 522: 349-355.
Singh NA et al (1998) Nature Genetics 18: 25-29.
Tinel N et al (1998) FEBS Lett 438: 17 J-176.
Tinel N et al (2000) FEBS Lett 480: J 37-141.
Wang H-S et al (1998) Science 282: 1890-1893.
Wang HS et al (2000) Mol Pharmacol 57: J 218-1223.
Yang WP et al (1997) Proc Nat Acad Sci USA 94: 4017-4021.
