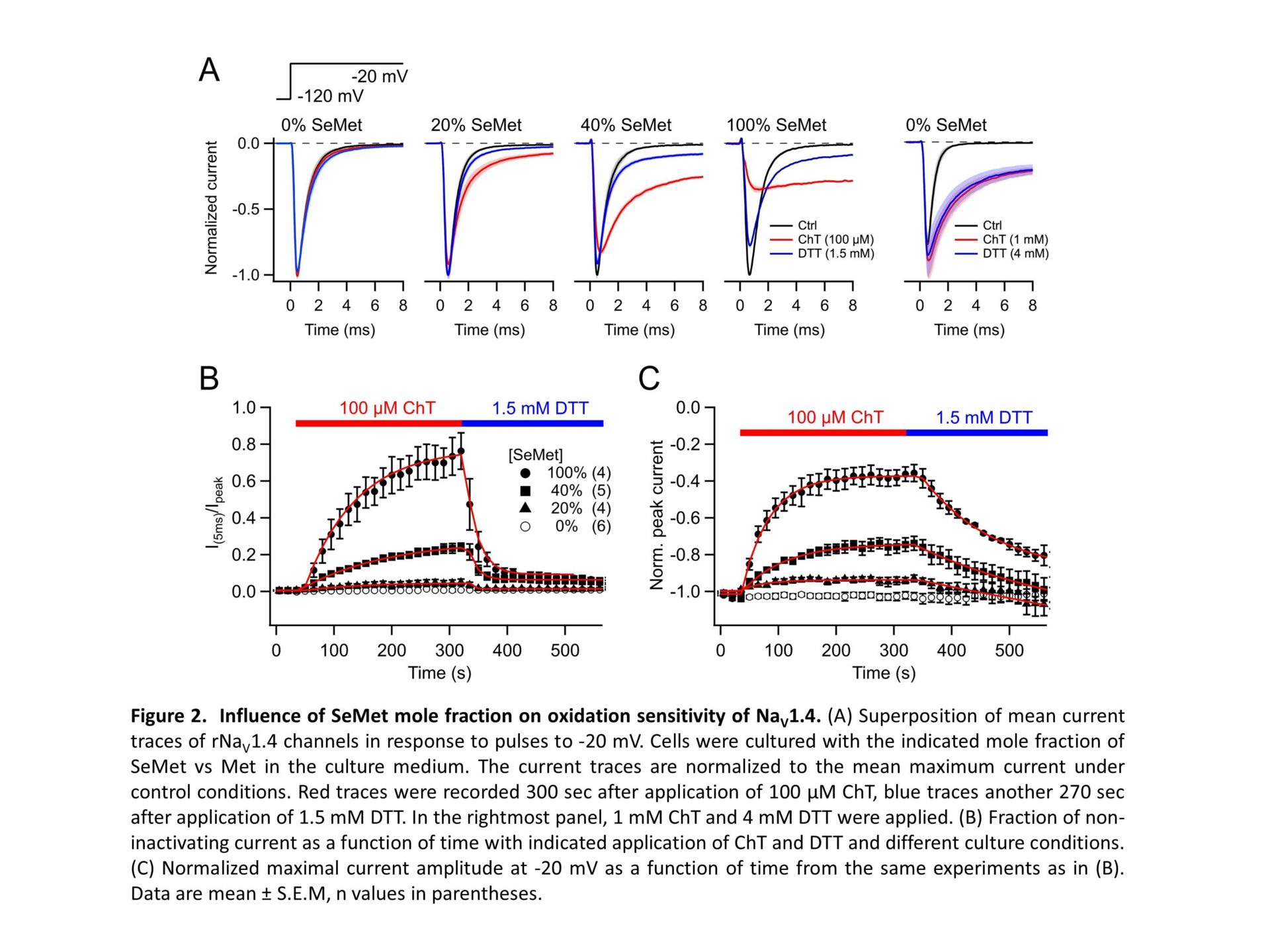
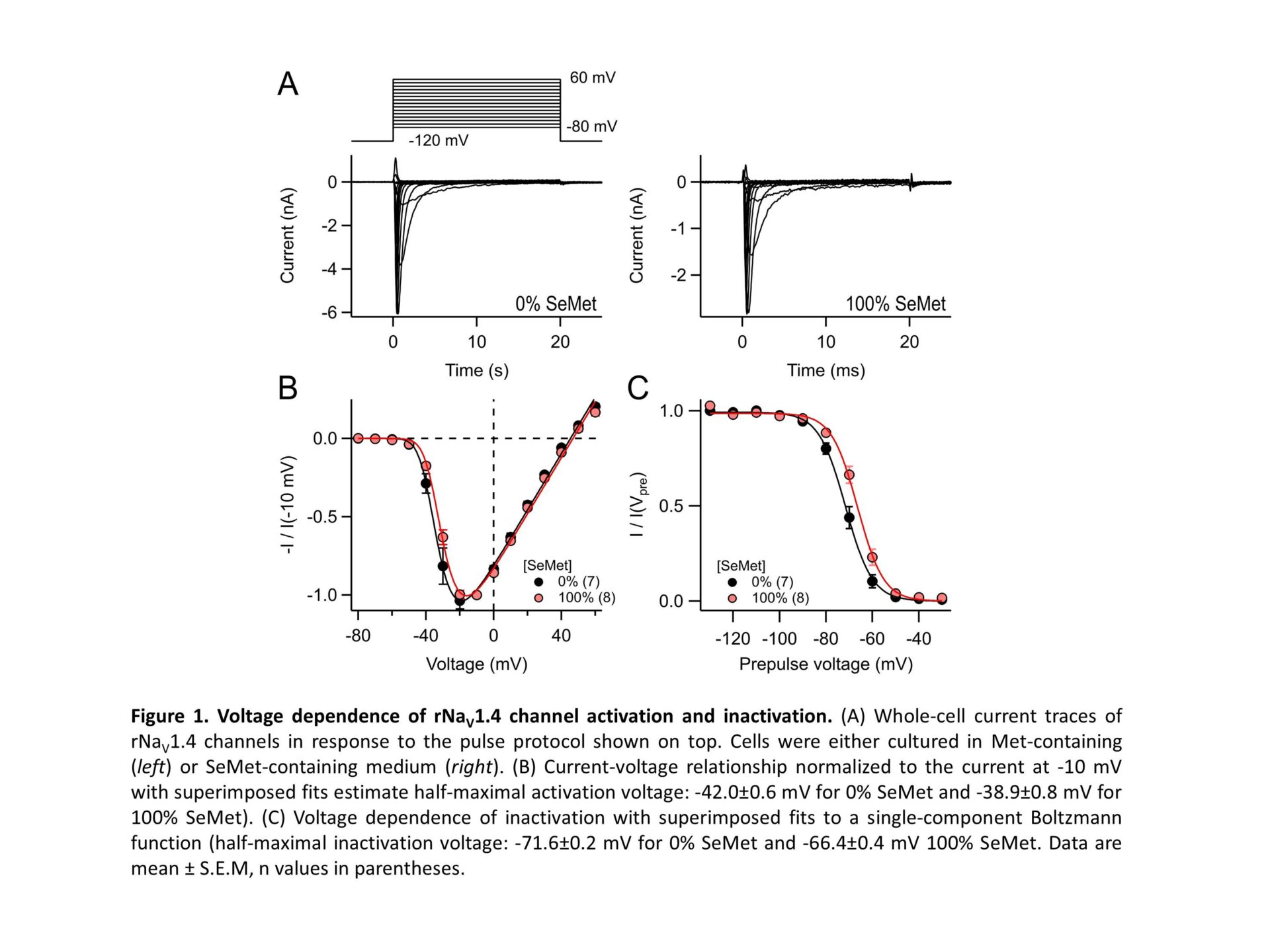
Selenomethionine (SeMet) mis-incorporation is a process where SeMet replaces methionine (Met) residues in proteins due to the high structural similarity between the two amino acids. The rate of mis-incorporation is dependent on the availability of SeMet to the protein synthesis machinery. SeMet content can reach to 90% of total selenium content in some plants consumed by humans. Moreover, SeMet is a supplement of selenium, an essential micronutrient with cancer chemo-preventive properties. SeMet gets readily absorbed via the methionine intestinal transporters and contributes to the methionine pool in the liver. Therefore, long-term intake of high SeMet doses results in the accumulation of the amino acid in muscle, heart and liver tissue proteins (Burk et al. 2015). Despite the high rates of incorporation of SeMet in proteins and its stronger susceptibility to oxidation compared to methionine, little is known about the effect of SeMet mis-incorporation on electrical excitability and ion channels. Fast inactivation of voltage-gated sodium channels (NaV) is essential for proper action potential generation, and even minute impairment of inactivation can result in adverse phenotypes. Surprisingly, NaV inactivation depends on the Ile-Phe-Met motif in the DIII-IV linker because oxidation of that Met results in marked loss of inactivation (Kassmann et al. 2008). Here we examined the impact of SeMet mis-incorporation on the function of skeletal muscle NaV1.4 channels. HEK293T cells were transfected with DNA coding for rat NaV1.4. Subsequently, cells were cultured in media supplemented with varying mole-fractions of Met vs SeMet (total concentration 200 µM). After about 24 h, currents were measured in the whole-cell patch-clamp configuration. In 100% SeMet medium, where almost all Met residues in a protein are expected to be replaced with SeMet, NaV1.4 channels (which harbors 66 Met residues) exhibited normal activation and inactivation properties with a slight right-shift in voltage dependence (Fig. 1). While 300-s exposure to 100 µM of the Met-specific oxidant chloramine T (ChT) had almost no impact on rNaV1.4 channels under control conditions, rNaV1.4SeMet channels responded with an almost complete loss of inactivation and about 60% reduction of the peak current amplitude at -20 mV (Fig. 2). Partial substitution of SeMet in culture media resulted in a graded response. The impact of oxidation on rNaV1.4SeMet was reversible with 1.5 mM dithiothreitol (DTT), while loss of inactivation on control channels induced by higher ChT concentration persisted even after DTT application. This observation is an indirect proof for SeMet being incorporated in NaV1.4 because oxidation of SeMet to methionine selenoxide is spontaneously reversible (Carroll et al. 2017). Great part of the redox sensitivity of NaV1.4SeMet channel inactivation can be attributed to the IFM motif because mutation M1305L (resulting in IFL) diminished the fraction of non-inactivating current from 0.77±0.09 (wild type) to 0.12±0.02 (n = 4-5). The molecular origin for the current amplitude reduction remains to be determined. SeMet incorporation in NaV1.4 channel proteins coinciding with oxidative insults may affect electrical excitability of neurons and myocytes to result in hyperexcitability pathologies, such as cardiac arrhythmias and neuropathies, similarly to congenital NaV channel gain-of-function mutations.
Physiology 2021 (2021) Proc Physiol Soc 48, OC21
Oral Communications: Selenomethionine mis-incorporation results in redox-dependent NaV1.4 channel gain-of-function
Rama Hussein1, Marwa Ahmed1, Stefan H. Heinemann1
1 Center of Molecular Biomedicine, Department of Biophysics, Friedrich Schiller University Jena and Jena University Hospital, Jena, Germany
View other abstracts by:
Where applicable, experiments conform with Society ethical requirements.