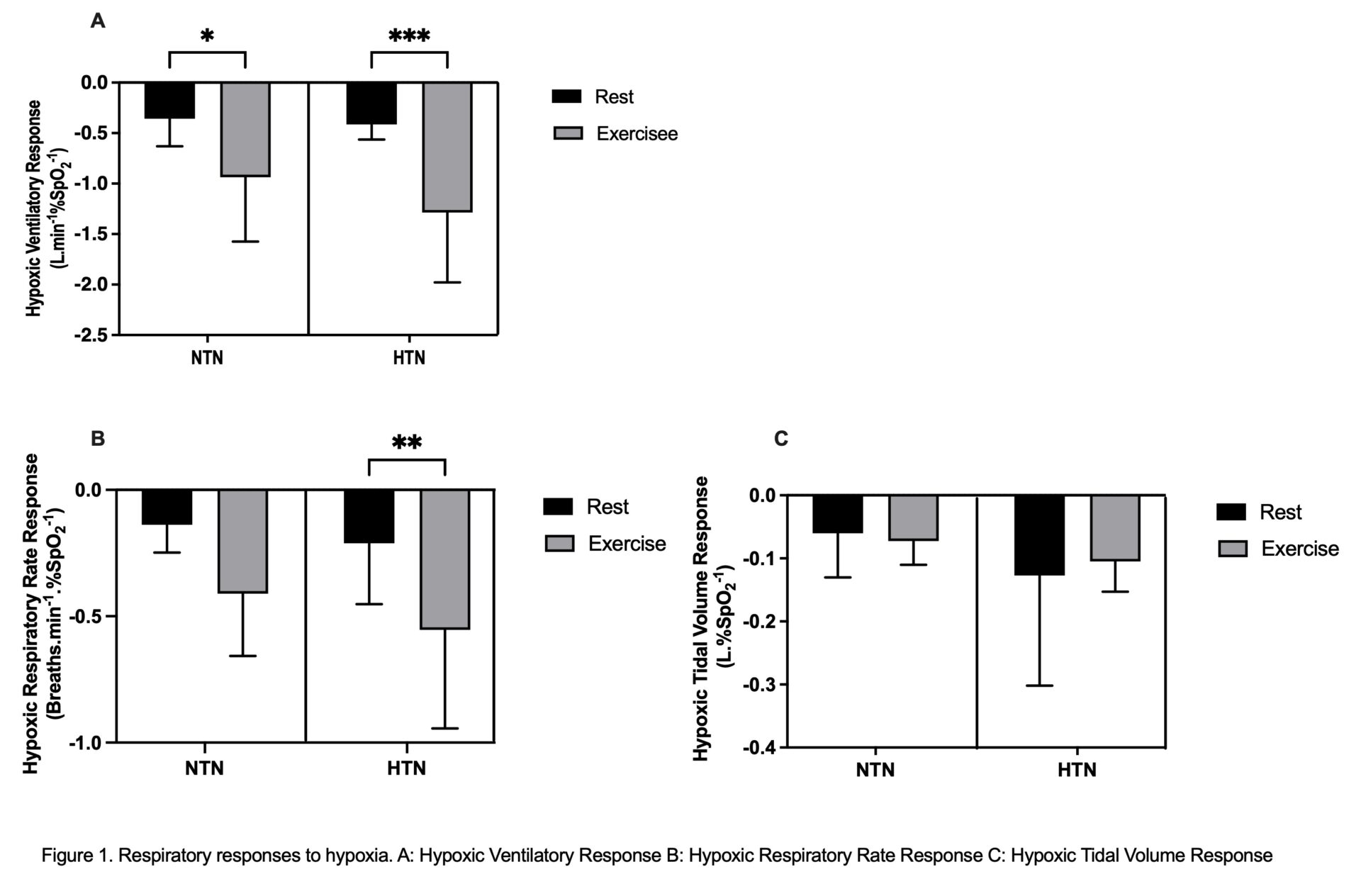
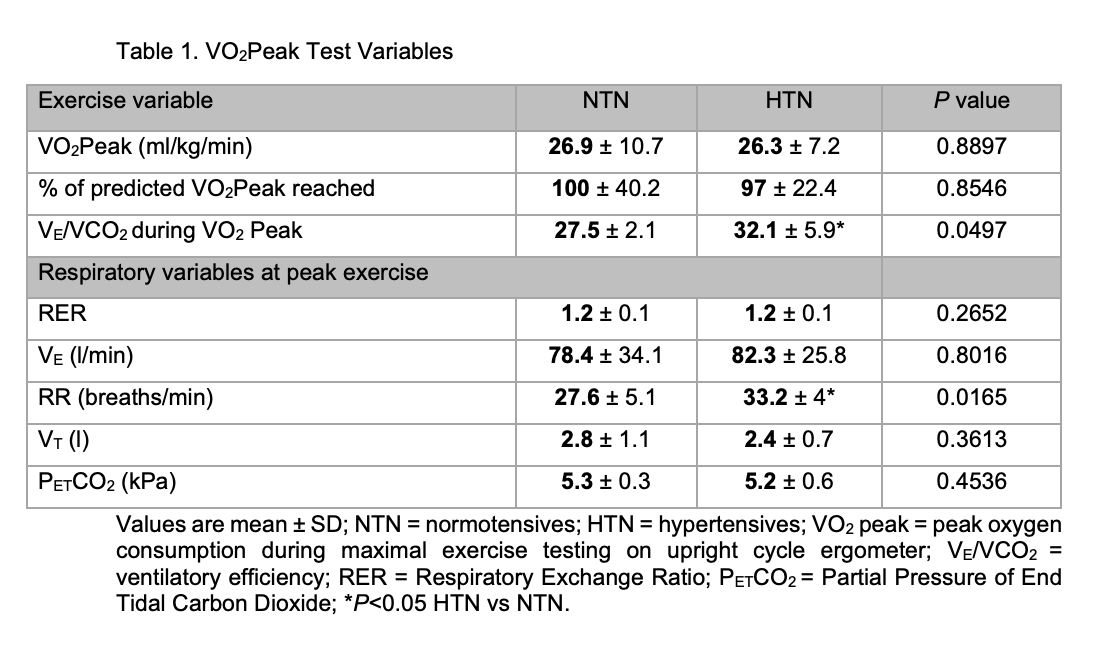
Hypertension is the single risk factor leading to the greatest number of directly attributable deaths worldwide (Murray et al., 2020). The carotid body (CB), a highly vascular gland situated bilaterally at the bifurcation of the common carotid arteries, is the body’s primary oxygen sensor. It also modulates sympathetic tone and has been shown to be hyperactive at rest in autonomic diseases such as hypertension (McBryde et al., 2013) The CB plays a role in the control of ventilation during exercise in healthy humans (Wasserman et al., 1975), although the exact mechanisms of exercise hyperpnea are incompletely understood. We aimed to assess CB chemosensitivity in human hypertension during submaximal exercise & gain insights into its role in exercise hyperpnea. All study protocols were approved by Frenchay South West National Research Ethics Service Committee (15/SW/0030) and conformed to the Declaration of Helsinki. We compared 12 hypertensive (HTN) patients (7 men; mean±SD age 65.3±7.6 years; 10 on anti-hypertensive therapy) to 8 healthy, normotensive (NTN) controls (6 men; 61±7.9 years). We assessed CB chemosensitivity as Hypoxic Ventilatory Response (HVR) in both groups at rest then during submaximal exercise (≈45% VO2peak, assessed previously using a standard protocol on an upright cycle ergometer). A poikilocapnic, hypoxic technique was used, targeting oxygen saturation (SpO2) levels of 87% for 2-3mins by the addition of nitrogen into inspired air. HVR was calculated using the method described by (Goldberg et al., 2017), such that the change in ventilation is divided by the change in SpO2. The same method was used to calculate Hypoxic Respiratory Rate (RR) and Tidal Volume (VT) Responses. Responses to hypoxia were analysed using 2-way ANOVA’s, with Sidak’s multiple comparison’s test. Peak exercise data were compared using unpaired T tests. Alpha was set at P<0.05. Data are reported as mean±SD. VO2Peak test variables are seen in Table 1. While VO2Peak was similar between groups, ventilatory efficiency was lower and peak RR higher in HTN vs NTN. The respiratory responses to hypoxia are seen in Figure 1. HVR was increased to the same extent from rest during exercise in both NTN and HTN (P <0.0001; Figure 1A). Hypoxic RR response was also increased overall by exercise (P = 0.0015) but with post hoc analysis showing only an increase in HTN (Figure 1B). There was no change in Hypoxic VT Response with exercise in either group (P = 0.8633; Figure 1C). These results indicate that there is no difference in HVR at rest and during exercise between HTN and NTN. HVR did increase from rest to exercise in both groups showing that exercise sensitizes the hypoxic chemoreflex control of ventilation. Interestingly, during exercise, the increase in HVR appears to be driven by RR (rather than VT) in HTN but not in NTN. The HTN group had a higher RR at maximal exercise versus controls. Potentially, inappropriate increases in RR during exercise are driven by the CB and may drive some ventilatory inefficiency in patients with hypertension. More work is needed to investigate this.
Physiology 2021 (2021) Proc Physiol Soc 48, PC034
Poster Communications: Carotid body chemosensitivity at rest and during exercise in patients with hypertension
Katrina Hope1, Angus K. Nightingale1, Julian Paton2, Emma C. Hart1
1 Universtiy of Bristol, Bristol, United Kingdom 2 The University of Auckland, Auckland, New Zealand
View other abstracts by:
Where applicable, experiments conform with Society ethical requirements.