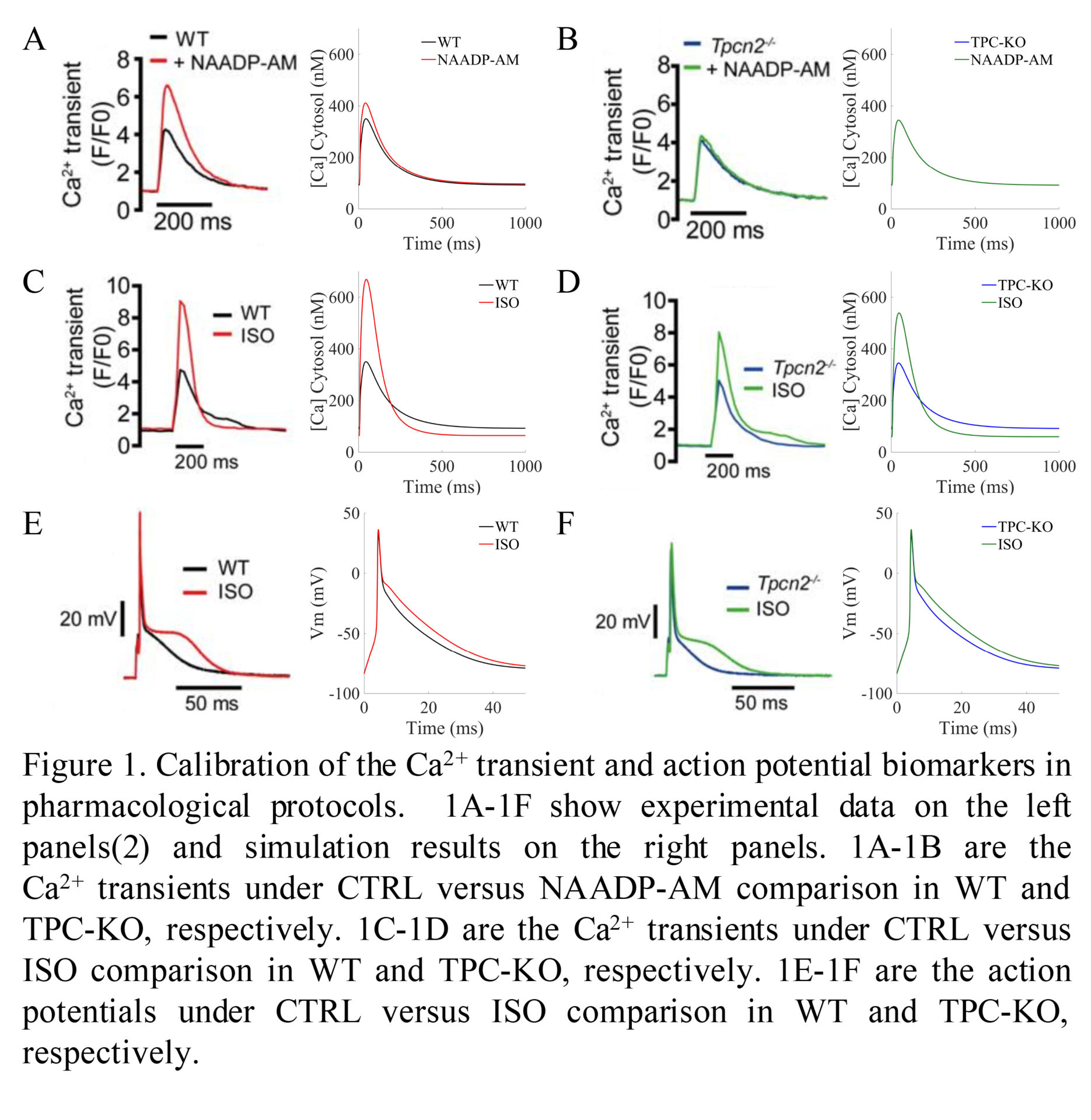
Background: Dysregulation of calcium handling is a well-recognised precursor of cardiac arrhythmias. Lysosomes have recently been shown to play an important role in cardiomyocyte calcium handling through calcium release via two-pore channels (TPC2)(1). Importantly, TPC2 knock-out (TPC2-KO) was found to markedly decrease the effects of β-adrenergic stimulation on calcium transient compared to wild type (WT) cardiomyocytes. These findings accounted for a reduced tendency for arrhythmias following acute β-adrenergic stimulation in comparison of TPC2-KO versus WT(2). The mechanisms underlying lysosomal calcium handling response to sympathetic stimulation remain unknown. Purpose: To investigate the key mechanisms underlying the lysosomal calcium handling response to β-adrenergic stimulation and its links to arrhythmogenesis using integrated experimental and computational methodologies. Methods: State-of-the-art computational models of mouse ventricular electrophysiology and β-adrenergic stimulation(3) were extended to incorporate lysosomal calcium handling, based on organelle localisation and action. Latin hypercube sampling was used to build an initial population of 1000 candidate models, to represent cellular variability in electrophysiology and calcium handling. Simulated action potentials and calcium transients were calibrated against experimental steady-state recordings for model acceptance in the final population. Accepted WT and TPC2-KO models were simulated in control (CTRL), under pharmacological action of the calcium-mobiliser messenger NAADP-AM, and β-adrenergic stimulation by isoproterenol (ISO). Influence of lysosomal action on calcium transients was quantified by analysis of distributions and multivariate partial correlation coefficients. Results: Data shown as mean ± SD. The initial population of models was calibrated against experimental recordings, resulting in a population of 605 accepted models. In agreement with experimental findings (Fig 1), no differences were observed in calcium transient between WT and TPC2-KO cardiomyocyte models under control conditions, whereas WT but not TPC2-KO responded to stimulation by NAADP-AM. The normalised calcium transient response under ISO stimulation was greater in WT (simulated: 1.02 ± 0.57; experimental: 1.23 ± 0.24) than TPC2-KO (simulated: 0.62 ± 0.37; experimental: 0.57 ± 0.17). These effects were mediated by a larger sarcoplasmic reticulum (SR) release and calcium content, primarily modulated by a potentiated SR Ca2+-ATPase (SERCA) reuptake due to the release of lysosomal calcium. Importantly, lysosomal calcium fluxes to the cytosolic space contributed significantly to modulation of calcium transient relaxation under β-adrenergic stimulation, surpassing the contribution of the L-type calcium current. The propensity for DAD formation as shown in the incidence of DADs in Ca2+ transients under hypercalcemia in β-adrenergic stimulation was increased in WT (62%) compared to TPC2-KO (48%). Conclusion: We have extended the existing Morotti et al.(2) model to include lysosomal calcium handling. Our results recapitulate experimental calcium transient findings of lysosomal calcium release in WT and genetically-modified TPC2-KO cardiomyocytes(2). These data show the impact of lysosomal calcium content in modulating intracellular calcium handling by increasing cytosolic calcium, which in turn promotes SERCA uptake, increases the SR calcium content, and potentiates Ca2+-induced Ca2+-release via ryanodine receptors. This increase in SR calcium content also potentially leads to the observed DAD formation. Our integrated experimental and computational methodology aims to elucidate the potential role of lysosomal calcium signalling in the mechanisms that underly arrhythmia.
Physiology 2021 (2021) Proc Physiol Soc 48, PC006
Poster Communications: Lysosomal calcium release modulates β-adrenergic response and arrhythmogenicity in ventricular cardiomyocytes by increasing SERCA activity
Zhao Meng1, Rebecca A Capel1, Samuel J Bose1, Alfonso Bueno-Orovio2, Rebecca A.B. Burton1
1 Department of Pharmacology, University of Oxford, Oxford, United Kingdom 2 Department of Computer Science, University of Oxford, Oxford, United Kingdom
View other abstracts by:
Where applicable, experiments conform with Society ethical requirements.