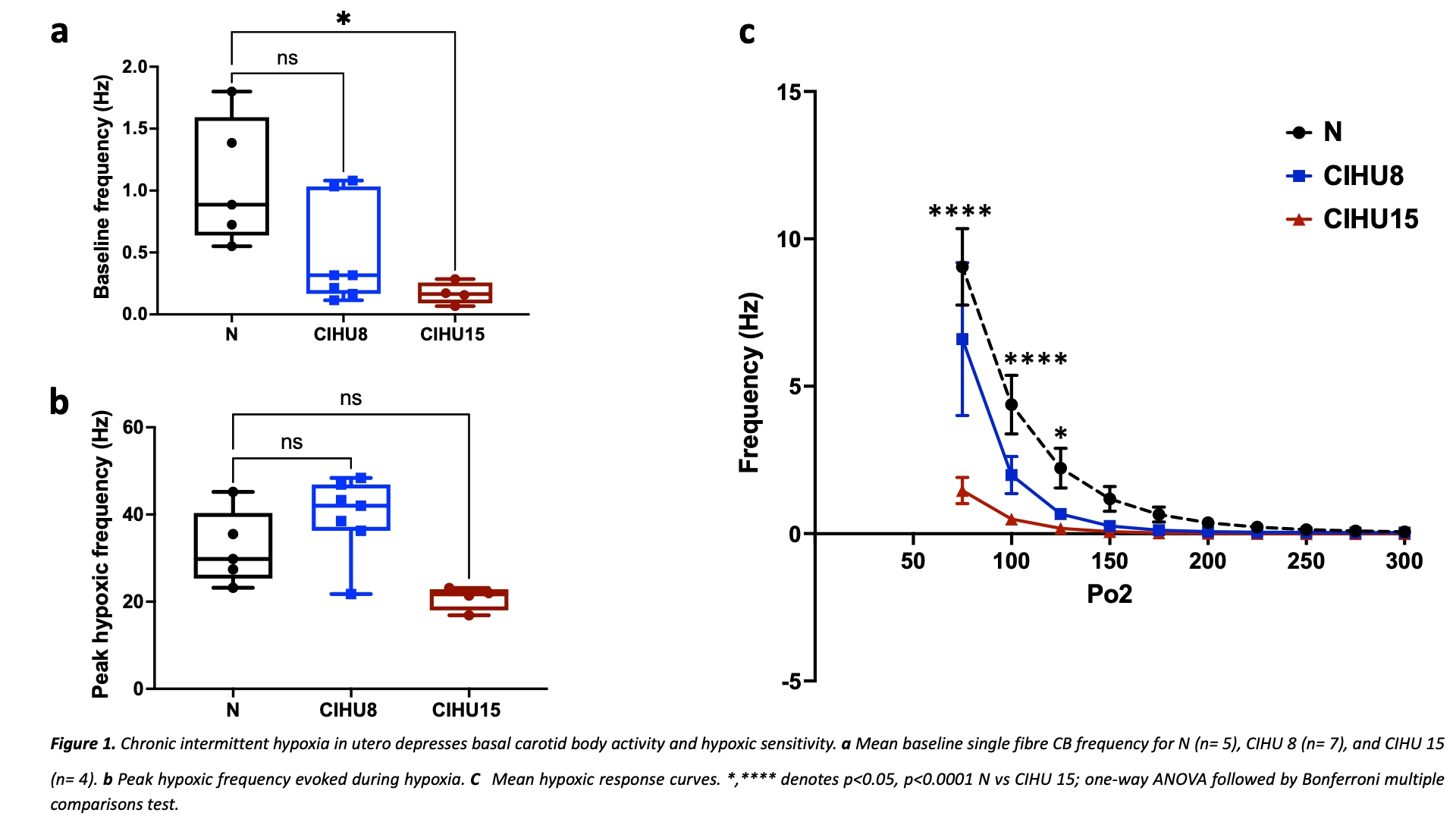
Introduction: Chronic intermittent hypoxia (CIH) is a common feature of obstructive sleep apnoea (OSA) and is prevalent in pregnancy, thus exposing the fetus to CIH [1]. In adults exposed to CIH, neurogenic hypertension develops as a consequence of carotid body (CB) hyperactivity. Evidence in animal models suggests that CIH in utero (CIHU) may cause subsequent hypertension in the adult [2]. Any role of the CBs in promoting hypertension development in adult offspring following CIHU programming is undefined. Aim: We aimed to identify whether CIHU alters basal CB activity and the hypoxic sensitivity in adult offspring, and if this is associated with alterations in cardiovascular and respiratory function. Methods: All animal procedures were approved by the Home Office (UK) (PPL number PF4C074AD) and University of Birmingham. 12 female Wistar rats (Charles River, UK) arrived on day 6 of pregnancy and were housed in individually ventilated cages with free access to food and water (n= 1 animal per cage). On day 10 of pregnancy, animals were randomly assigned to 3 different groups: normoxia (N; n= 4), mild maternal CIH (8 cycles/hour, 8 hours per day; CIHU 8; n=4), and severe maternal CIH (15 cycles/hour, 8 hours per day, CIHU 15; n=4). Following birth, male offspring were matured to adults (10-20W) without further exposure to hypoxia. The in vitro CB chemoafferent activity was assessed on freshly isolated CBs, in normoxic conditions (superfusate PO2 ca 300mmHg) and during hypoxia (PO2ca 100mmHg). Respiratory measurements were collected on awake animals using whole body plethysmography and cardiovascular reflex responses to hypoxia were performed under terminal anaesthesia (alfaxan i.v 17 – 20 mg kg-1 h-1), as described [3]. Results: Basal chemoafferent activity was significantly decreased in adult offspring exposed to severe but not mild CIHU (N 1.±0.2 vs. CIHU 15 0.2±0.04 Hz, P<0.05, Fig.1a). Similarly, the CB response to hypoxia was depressed in the animals exposed to severe CIHU, when measured at fixed, defined superfusate PO2s. E.g. At a superfusate PO2 of 100mmHg: N 4±1 vs. CIHU 15 0.5±0.15 Hz, P<0.0005 (Fig. 1c). Absolute peak chemoafferent responses were consistent between groups but occurred at lower PO2s in the CIHU animals, consistent with a reduced CB O2sensitivity. Interestingly, both mild and severe CIHU did not alter the mean arterial blood pressure in normoxia or hypoxia. Furthermore, baseline ventilation and the hypoxic ventilatory response were unaltered by CIHU. Conclusion: These results suggest that prenatal CIH leads to attenuation of CB function in the adult without completely abolishing it. However, this change did not alter the basal cardiovascular and respiratory measurements or attenuate reflex responses to hypoxia. The inconsistency of the current findings with the evidence that showed CIH in utero causes hypertension may be due to the lower CIH frequency and the duration used in this study compared to other studies. Further adaptations in the chemoreflex may also act to counter this reduction in the CB activity, preventing systemic cardiorespiratory dysfunction- future experiments are needed to explore this further.
Physiology 2021 (2021) Proc Physiol Soc 48, PC022
Poster Communications: Chronic intermittent hypoxia in utero depresses basal carotid body activity and hypoxic sensitivity in adult offspring
Abdulaziz A. Alzahrani1, 2, Andrew P. Holmes1, 3, Hayyaf S. Aldossary1, 4, Agnieszka Swiderska5, Clare J Ray1, Prem Kumar1, Andrew M. Coney1
1 1. Institute of Clinical Sciences, University of Birmingham, Birmingham, B15 2TT, UK., Birmingham, United Kingdom 2 2. Respiratory Care Department, Faculty of Applied Medical Sciences, Umm Al-Qura University, Makkah 24381, Saudi Arabia. , Makkah, Saudi Arabia 3 3. Institute of Cardiovascular Sciences, University of Birmingham, Edgbaston, Birmingham, UK, B15 2TT., Birmingham, United Kingdom 4 4. College of Medicine, Basic Medical Sciences, King Saud bin Abdulaziz University for Health Sciences, Riyadh, 11481, Saudi Arabia., Riyadh, Saudi Arabia 5 5. Unit of Cardiac Physiology, Institute of Cardiovascular Sciences, Manchester Academic Health Sciences Centre, The University of Manchester, 3.26 Core Technology Facility, 46 Grafton Street, Manchester M13 9NT, UK., Manchester, United Kingdom
View other abstracts by:
Where applicable, experiments conform with Society ethical requirements.