Our knowledge of inositol trisphosphate (IP3) as a calcium mobilizing second messenger derives primarily from studies in non-excitable cells. In contrast, less is known regarding its functions in neurons, despite the important roles played by cytosolic Ca2+ ions in regulating electrical excitability and synaptic plasticity (Berridge, 1998). Most studies of neuronal IP3 signalling have focused on the cerebellum and hippocampus, and surprisingly little is known about its functions in the cerebral cortex. In this study, we analysed neurons in the prefrontal cortex, where IP3-evoked Ca2+ signals are likely to exert widespread influences on sensory, motor, limbic and memory systems.
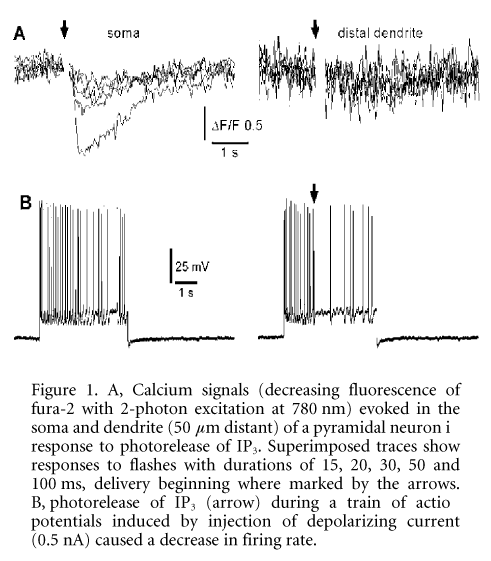
We combined in vitro visualized whole-cell electrophysiological recordings with 2-photon, video-rate Ca2+ imaging (Nguyen et al. 2001). Layer V pyramidal neurons from mouse brain slices were filled with the Ca2+ indicator fura-2 (50 mM) and caged IP3 (50 mM). Mice were deeply anaesthetized with halothane before decapitation, in compliance with UCI IACUC regulations. Ca2+ responses following photorelease of intracellular IP3 by flashes of UV light could be categorized into the following groups: (1) non-responders (no Ca2+ release even with strong UV flashes, 31 %, 33/106 neurons); (2) slow and weak responders (17 %); (3) brisk responders (rapid and large Ca2+ increase with short UV flashes, 56 %). In all responding cells, the soma demonstrated the highest sensitivity to IP3, while release in the dendrites required either much stronger flashes, or was absent altogether (Fig. 1A). Interestingly, IP3-evoked Ca2+ signals in many non- and weakly responding cells could be ‘rescued’ by evoking a train of action potentials prior to uncaging IP3. In contrast, paired UV flashes in responders resulted in suppression of the second Ca2+ response. Voltage-clamp studies revealed the activation of a slow outward current following photorelease of IP3, and UV flashes during a train of action potentials evoked by injection of inward current resulted in suppression of spikes (Fig. 1B).
These data demonstrate bi-directional interactions between intracellular IP3-evoked Ca2+ release and influx of extracellular Ca2+ through voltage-gated channels in cortical pyramidal neurons. Ca2+ influx potentiates IP3-mediated signals via mechanisms involving both a co-agonist effect on the IP3 receptor and enhanced filling of IP3-sensitive stores. Conversely, cytosolic Ca2+ elevations evoked in the soma by liberation from IP3-sensitive stores powerfully reduce neuronal excitability by activating Ca2+-dependent K+ conductances.
This work was supported by the NIH. G.E.S. is supported by an NIA training grant.
All procedures accord with current local guidelines