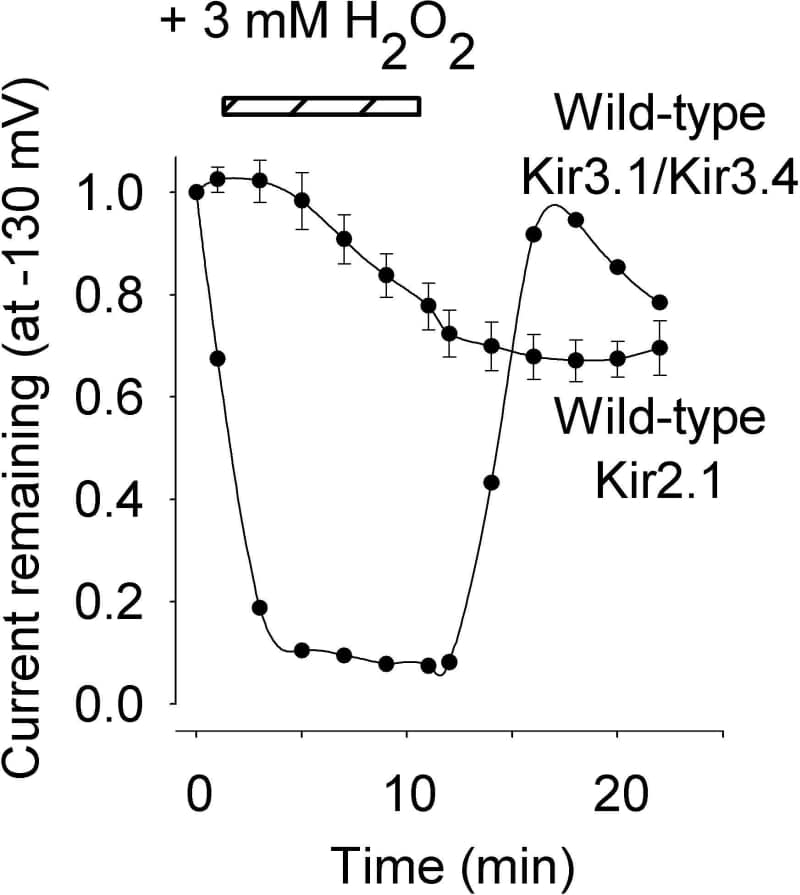
Kir3.1/Kir3.4 and Kir2.1 are inward rectifier K+ channels underlying IK,ACh (ACh-activated K+ current) and IK,1 (background K+ current) in the heart. Reactive oxygen species such as H2O2 are implicated in ischemia/reperfusion injury. We have compared the effect of H2O2 on Kir3.1/Kir3.4 and Kir2.1 channels expressed in Xenopus oocytes. Currents were recorded using the two-electrode voltage clamp technique during 750 ms voltage clamp pulses from -130 to +60 mV from a holding potential of 0 mV. Perfusion of 3 mM H2O2 for 10 min resulted in a substantial inhibition of Kir3.1/Kir3.4 current but not Kir2.1 current (Fig. 1). Kir3.1/Kir3.4 current was inhibited by 92.3±1.2%, but Kir2.1 current was inhibited by 16.3±4% (mean±SEM; n=5; Kir3.1/Kir3.4 vs Kir2.1, P<0.001, t test). Kir3.1/Kir3.4 current showed reversible inhibition. However, Kir2.1 current showed irreversible inhibition. The reversible inhibition of Kir3.1/Kir3.4 by H2O2 argues against irreversible oxidation of the Kir3.1/Kir3.4 channel and suggests another possible mechanism of action. The Kir3.1/Kir3.4 channel has been shown to be suppressed by tyrosine phosphorylation (Rogalski et al. 2000) and H2O2 activates tyrosine kinases (Yan & Berton, 1996). Endogenous insulin-like growth factor receptor activation with 2 μM insulin (expected to activate tyrosine kinases) and application of 100 μM 4 methoxyphenacyl Br (tyrosine phosphatase inhibitor) inhibited Kir3.1/Kir3.4 current by 45.0±22.1% and 46.9±6.0%, respectively (n=5). Oocytes expressing the Kir3.1/Kir3.4 channel were incubated with 3 mM H2O2 and then homogenised and the detergent soluble fraction was taken for immunoprecipitation with anti-Kir3.1 antibodies. Immunoblotting of immunoprecipitates with anti-phosphotyrosine and anti-Kir3.1 antibodies confirmed the presence of tyrosine phosphorylated Kir3.1 subunits. These observations demonstrate that Kir3.1/Kir3.4 and Kir2.1 channels respond differently to H2O2. It is possible that Kir3.1/Kir3.4 is regulated by H2O2 via tyrosine phosphorylation. However, Kir2.1 is also known to be inhibited by tyrosine phosphorylation (Wischmeyer et al. 1998). Another explanation is that Kir3.1/Kir3.4 is regulated by H2O2 via reversible oxidation of reactive cysteine residues (Zeidner et al. 2001).
University of Bristol (2005) J Physiol 567P, PC133
Poster Communications: A difference in H2O2 inhibition of the Kir3.1/Kir3.4 and Kir2.1 inward rectifier K+ channels
Makary, Samy M.Y.; Leach, Rob N; Boyett, Mark R;
1. Medicine, Manchester University, Manchester, United Kingdom. 2. School of Biochemistry and Molecular Biology, University of Leeds, Leeds, United Kingdom.
View other abstracts by:
Figure 1. Effect of H2O2 on wild-type Kir3.1/Kir3.4 and Kir2.1 channels. Data are means ± S.E.M. (n=5).
Where applicable, experiments conform with Society ethical requirements.