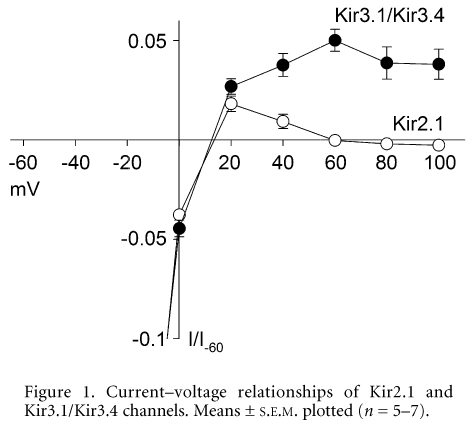
Kir2.1 and Kir3.1/Kir3.4 are inwardly rectifying K+ channels. We have compared the extent of inward rectification in these channels. Chinese hamster ovary (CHO) cells were transfected with cDNA encoding Green Fluorescent Protein and Kir2.1 or Kir3.1/Kir3.4 and currents were recorded 24-72 h later using the whole-cell patch-clamp technique: 750 ms voltage pulses from -60 to +100 mV were applied from a holding potential of 0 mV at 0.33 Hz. Data were obtained with 140 mM K+ in both the pipette and bathing solutions. Figure 1 shows normalised current-voltage relationships for Kir2.1 and Kir3.1/Kir3.4. There was less inward rectification in Kir3.1/Kir3.4 than Kir2.1: whereas outward current through Kir2.1 reduced to negligible levels at potentials greater than +60 mV, outward current through Kir3.1/Kir3.4 persisted.
Inward rectification involves occlusion of the channel pore by intracellular polyamines, such as spermine. We have investigated the effect of extracellular spermine on Kir2.1 and Kir3.1/Kir3.4 channels expressed in Xenopus oocytes. cRNA encoding Kir2.1 or Kir3.1/Kir3.4 was injected into oocytes and currents recorded 16-96 h later using the two-electrode voltage-clamp technique; the voltage-clamp protocol was the same as above, but pulses were applied from -130 to +60 mV. By replacing extracellular K+ (90 mM) with spermine (90 mM), permeation of the polyamine through each channel was measured. Spermine could not permeate the Kir2.1 channel; sustained current at -130 mV was -0.4 ± 0.6 mA (n = 10) with spermine as the main charge carrier (not different from endogenous current; -0.2 ± 0.1 mA, n = 5; unpaired t test, n.s.) compared with -14.2 ± 5.2 mA with K+ as the main charge carrier. In contrast, substantial inward current through the Kir3.1/Kir3.4 channel was measured with spermine as the main charge carrier, suggesting that spermine can permeate the Kir3.1/Kir3.4 channel; current was -2.7 ± 0.6 mA (n = 6) with spermine (significantly different from endogenous current; unpaired t test, P < 0.05) compared with -11.3 ± 1.3 mA with K+ as the main charge carrier. These data show that there is less inward rectification in the Kir3.1/Kir3.4 channel than the Kir2.1 channel and we suggest that this is because spermine can permeate the Kir3.1/Kir3.4 but not the Kir2.1 channel.
Interestingly, K+ current through the Kir3.1/Kir3.4 channel was blocked by extracellular spermine (1-30 mM; 90 mM extracellular K+ present) more effectively than K+ current through the Kir2.1 channel; the affinity of spermine for the Kir3.1/Kir3.4 channel was approximately 28-fold greater (at -130 mV; n = 6-10) than for the Kir2.1 channel.
This work was supported by the British Heart Foundation.
All procedures accord with current UK legislation.