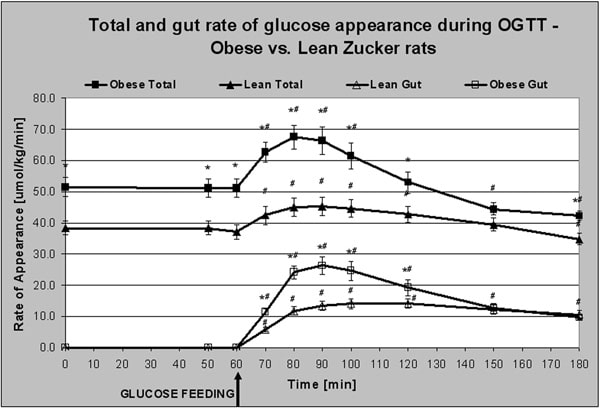
Obesity and insulin resistance (IR) are both part of the Metabolic Syndrome and have developed into world-wide epidemics. The demand for drugs that improve glycemic control after a meal is high and will increase in future years. Many of the current drugs are being tested for their potential metabolic action in rodents, both in healthy controls and in models of obesity and IR. However, the existent methodology to quantitate glucose and fat metabolism in animal models of IR is limited. The aim of this study was to quantify glucose and fat metabolism in obese Zucker rats, a model of IR, using a downscaled version of a dual stable isotope tracer method that is currently used in man. Glucose and fat metabolism were measured in obese and lean Zucker rats after an overnight fast and under general anaesthesia. Animals were placed in an open-flow indirect calorimeter chamber to determine oxidation rates of glucose and fat. Two catheters were in place for intravenous infusion of [6,6-2H2]glucose and anaesthetic. A glucose solution enriched with [U-13C]glucose was administered into the stomach by gavage. Blood was sampled from the left carotid artery. Whole-body insulin sensitivity was calculated from glucose and insulin levels during a 2 hour oral glucose tolerance test (OGTT) as described in [1]. Non-steady state glucose kinetics were calculated using Steele’s single pool model and the calculations described in [2] during the OGTT. Table 1 shows that obese Zucker rats were highly insulin resistant compared with lean controls. Area under the curve (AUC) of plasma insulin after glucose ingestion was higher in obese rats, but no difference in glucose-AUC was measured. Total and gut glucose rate of appearance were higher in the obese animals in the fasted state and at most times during the OGTT (Fig 1). However, no difference in suppression of hepatic glucose output was found (-27.8%±3.3 v -20.6%±1.1) (P=0.07). The contribution of fat oxidation to total energy production was lower in obese rats in both the fasting state (60.8%±4.4 v 78.1%±1.7)* and during the OGTT (46.3%±3.2 v 66.1%±2.1)*#. No difference in the suppression of fat oxidation was measured (P=0.13). (*P<0.05 compared to lean; #P<0.05 compared to basal). We conclude that glucose and fat metabolism could successfully be quantified in anaesthetized obese Zucker rats using a downscaled version of a dual stable isotope tracer method. Future work will focus on the effect of known and novel hyperglycemic drugs. By using the same methodology as in man, compound efficacy with respect to glucose clearance and hepatic glucose production can now be investigated in animal models.
Life Sciences 2007 (2007) Proc Life Sciences, PC234
Poster Communications: A dual tracer method to quantify glucose metabolism in anaesthetized obese Zucker rats in vivo
E. Zijlstra1, 2, A. Wagenmakers1, R. Potter2, S. Poucher2
1. School of Sport and Exercise Sciences, The University of Birmingham, Birmingham, United Kingdom. 2. CVGI Discovery, AstraZeneca, Macclesfield, United Kingdom.
View other abstracts by:
Table 1
Figure 1
Where applicable, experiments conform with Society ethical requirements.