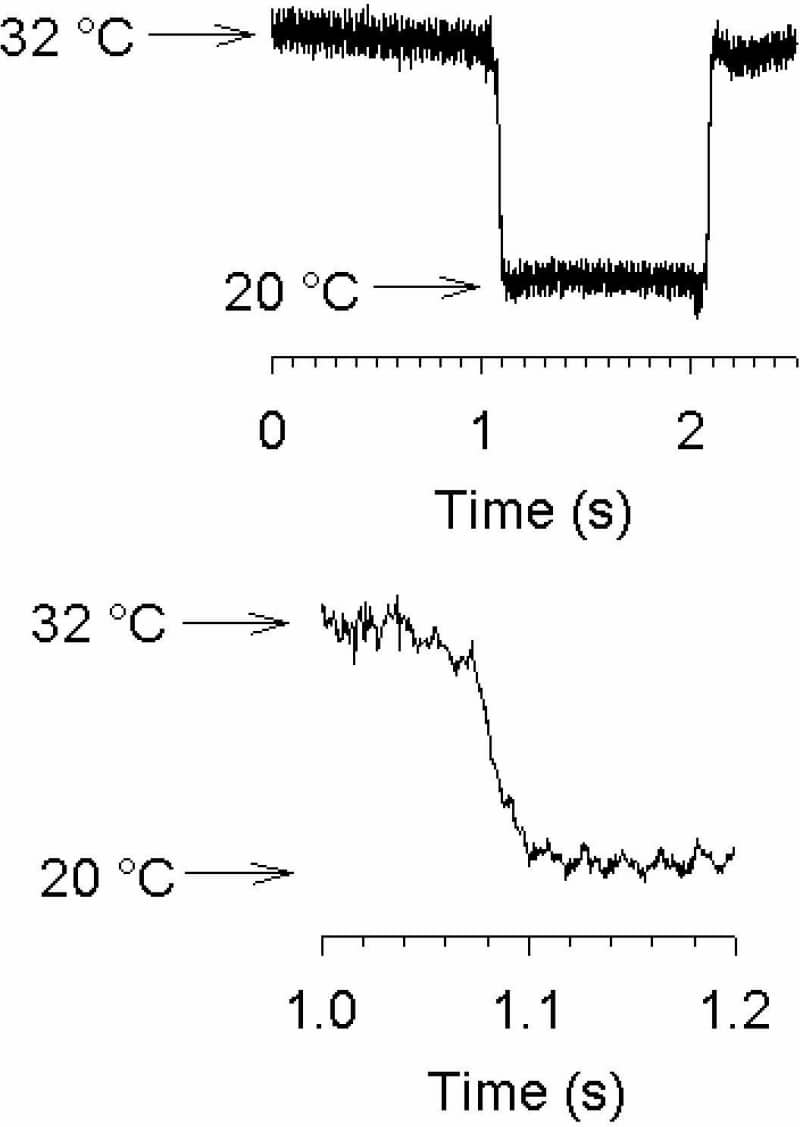
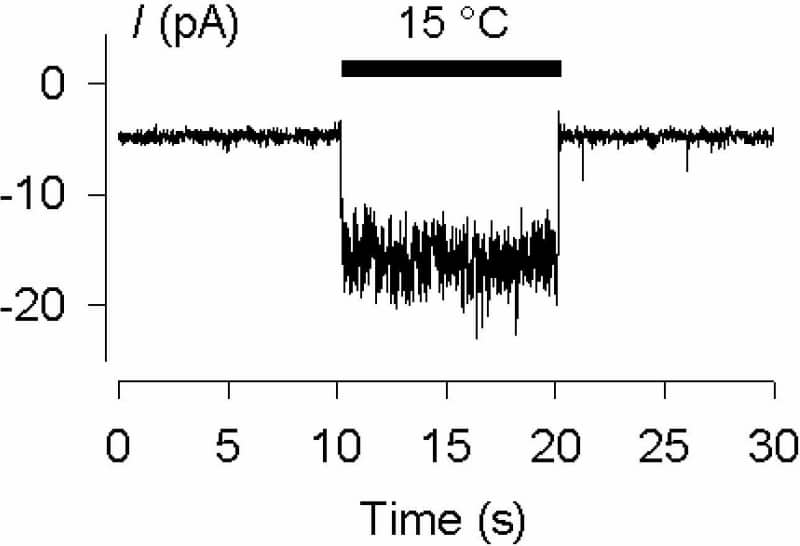
Commercially available systems for temperature control during patch clamp or [Ca2+]i measurements are generally designed to maintain a constant baseline temperature. Few systems are as yet available that allow temperature itself to be used as a stimulus. Some years ago we described a system allowing heating and cooling steps with a time constant of ~5 s, or ramps up to 4 °C/s, to be applied (Reid et al., 2001). This has proven useful in our own work and that of others, but the timecourse of the stimulus nevertheless limits the experiments that can be done. This limitation led to the development of the system presented here. A twin version of our earlier Peltier-based system, with each Peltier unit powered by an independent feedback controller, is connected to a commercial stepper motor unit (SF-77B; Warner Instruments, Hamden, CT). Solutions flow across the Peltier elements through stainless steel tubing (18 gauge; two tubes on each Peltier element) and into 1 mm square glass outlet tubes positioned close to the cell or membrane patch being recorded. The stepper motor moves the outlet tubes so that solutions that have flowed across one or the other Peltier element can be applied to the cell or patch. This allows rapid switching between any two independently controllable temperatures, and also between control and drug solutions at the same temperature. Switching time is typically ~20 ms between any two temperatures when 1 mm square tubing is used (Fig. 1). Theta glass allows faster switching times, but heat exchange across the thin glass septum limits the usefulness of this approach. To minimise heat exchange between the 1 mm square glass tubes we normally use, we mount the outlet tubes so that they converge at an angle of ~20° and meet only at the tip. As well as studies on the kinetics of temperature activation of thermoTRP channels (Fig. 2), the system described here has allowed us to identify a novel group of rapidly adapting cold-sensitive DRG neurones (see communication by Babes et al., this meeting).
University of Bristol (2005) J Physiol 567P, D12
Demonstrations: A rapid system for applying thermal stimuli during patch clamp and [Ca2+]i imaging experiments
Reid, Gordon; Zorzon, Daniel;
1. Faculty of Biology, University of Bucharest, Bucharest, Romania.
View other abstracts by:
Time course of switching between 32 °C and 20 °C measured using the change in offset potential at the tip of a patch clamp pipette (thermocouples and thermistors are too slow to track the fast switching). The switch from 32 °C to 20 °C is shown on an expanded timebase in the lower panel.
Rapid activation of the native cold and menthol receptor TRPM8 by a switch from 25 °C to 15 °C in a multi-channel excised patch.
Where applicable, experiments conform with Society ethical requirements.