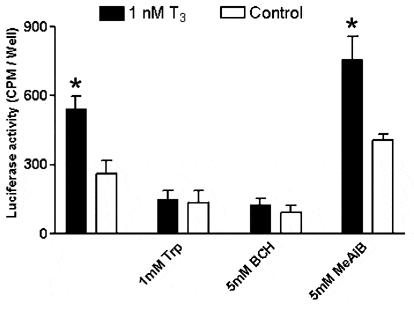
Thyroid hormones (TH; T4 and T3) regulate growth, development and metabolism by acting through nuclear TH-receptors. Plasma TH must reach the cell nucleus to exert their genomic effects. Recent evidence indicates that transport (rather than diffusion) mediates the greater part of TH movement across the plasma membrane. For example, we have shown that amino acid (AA) transport System L mediates TH uptake in certain cell types (e.g. BeWo placental cells; Ritchie & Taylor, 2001). We are investigating the possibility that TH transport by System L is an overlooked step for control of cellular TH action. Monolayer cultures of BeWo cells were incubated in NaCl transport buffer containing 100 pM 125I-T3 for 30 min followed by rapid processing into nuclear and cytosolic fractions. Blockade of System L using tryptophan (Trp; 10 mM) reduced cell uptake and nuclear binding of 125I-T3 to 48 ± 5 and 39 ± 6 %, respectively, of control value (mean ± S.E.M., n = 5 experiments) without disturbing nuclear binding kinetics. We next investigated whether these inhibitory effects on nuclear binding of T3 reflected reduced activation of TH-dependent gene transcription. A T3-responsive luciferase reporter (Menjo et al. 1999) introduced into BeWo cells via an adenovirus vector 24 h prior to experiments exhibited T3-dependent activation of transcription. This activation was suppressed in the presence of System L substrates BCH (2-amino [2, 2, 1] heptane-2-carboxylic acid) or Trp (Fig. 1), which would compete out T3 uptake into cells through this route. In contrast, N-methylamino-isobutyric acid (MeAIB; a specific competing substrate of AA transport System A) was without effect.
These results indicate that plasma membrane transport of T3 at physiologically relevant concentrations (100 pM) by system L is an important determinant of nuclear T3 entry and action in BeWo cells. System L is expressed ubiquitously in mammalian cells and may therefore have a widespread role in cellular TH signalling.
This work was supported by BBSRC, NIH and University of Dundee.