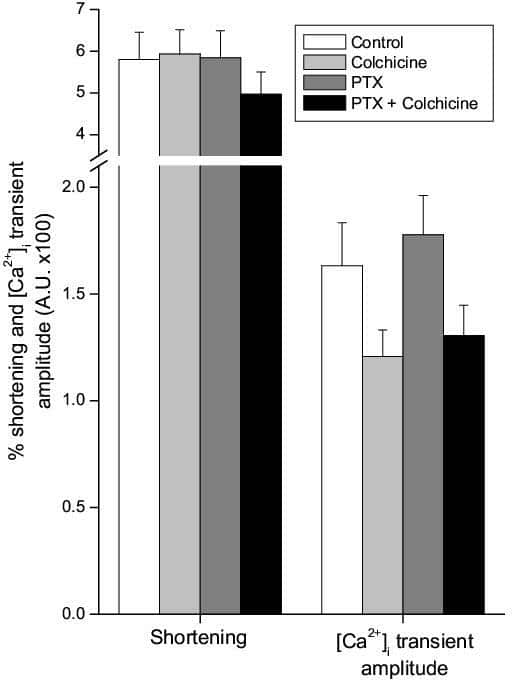
In neuronal tissue, free tubulin can activate both stimulatory (Gs) and inhibitory (Gi) G-protein α-subunits via transfer of GTP from the exchangeable GTP-binding site on β-tubulin (Rasenick et al. 1981). It is debatable whether such signalling occurs in the heart. Colchicine-induced microtubule disruption, which increases free tubulin, is reported to be without effect on shortening or [Ca2+]i transient amplitude in normal rat ventricular myocytes (Calaghan et al. 2001, 2004). In contrast, Kerfant et al. (2001) have shown an increase in [Ca2+]i transient amplitude after colchicine treatment in the same preparation. These workers suggested that Gs α-subunit stimulation of adenyl cyclase underlies increased [Ca2+]i transients after microtubule disruption. In this study we determine whether a predominance of the Gi pathway in our animal model is masking Gs responses that might increase contraction or [Ca2+]i transients after colchicine treatment. Male Wistar rats were killed humanely, and myocytes were isolated enzymatically from the right ventricle. Cells were incubated in 1.5 mg ml-1 pertussis toxin (PTX, a Gi α-subunit inhibitor) and/or 10 μM colchicine for at least 1.5 h in normal Tyrode solution at room temperature. Shortening (determined by video-edge detection) and [Ca2+]i transients were measured simultaneously in fura-2-loaded cells (37 °C, 1 Hz stimulation). Statistical analysis was performed using one-way ANOVA. The effect of colchicine and PTX on shortening and [Ca2+]i transient amplitude is shown in Fig. 1. Treatment with colchicine or PTX alone did not alter the shortening amplitude or the [Ca2+]i transient when compared with controls (P>0.05). Furthermore, co-incubation with PTX did not change the shortening or [Ca2+]i transient response to colchicine (P>0.05). PTX has no effect on shortening or [Ca2+]i transient characteristics nor does it modulate the response to colchicine. We conclude, therefore, that predominant Gi α-subunit signalling cannot explain the lack of effect of colchicine on contraction or [Ca2+]i transients under the conditions of our experiments. Our data are in agreement with a body of evidence showing that microtubule disruption by colchicine in the healthy cardiac cell is without functional consequences.
University of Bristol (2005) J Physiol 567P, PC13
Poster Communications: Abolition of G-protein inhibitory subunit signalling does not reveal a positive inotropic effect of microtubule disruption in rat ventricular myocytes
O'Connell, Anthony D; Calaghan, Sarah C; White, Ed;
1. School of Biomedical Sciences, Universiy of Leeds, Leeds, United Kingdom.
View other abstracts by:
Figure 1: The effect of PTX and/or colchicine on shortening (as a % resting cell length) and intracellular calcium transient amplitude in rat ventricular myocytes. Bars are means +SEM (n=36-41).
Where applicable, experiments conform with Society ethical requirements.