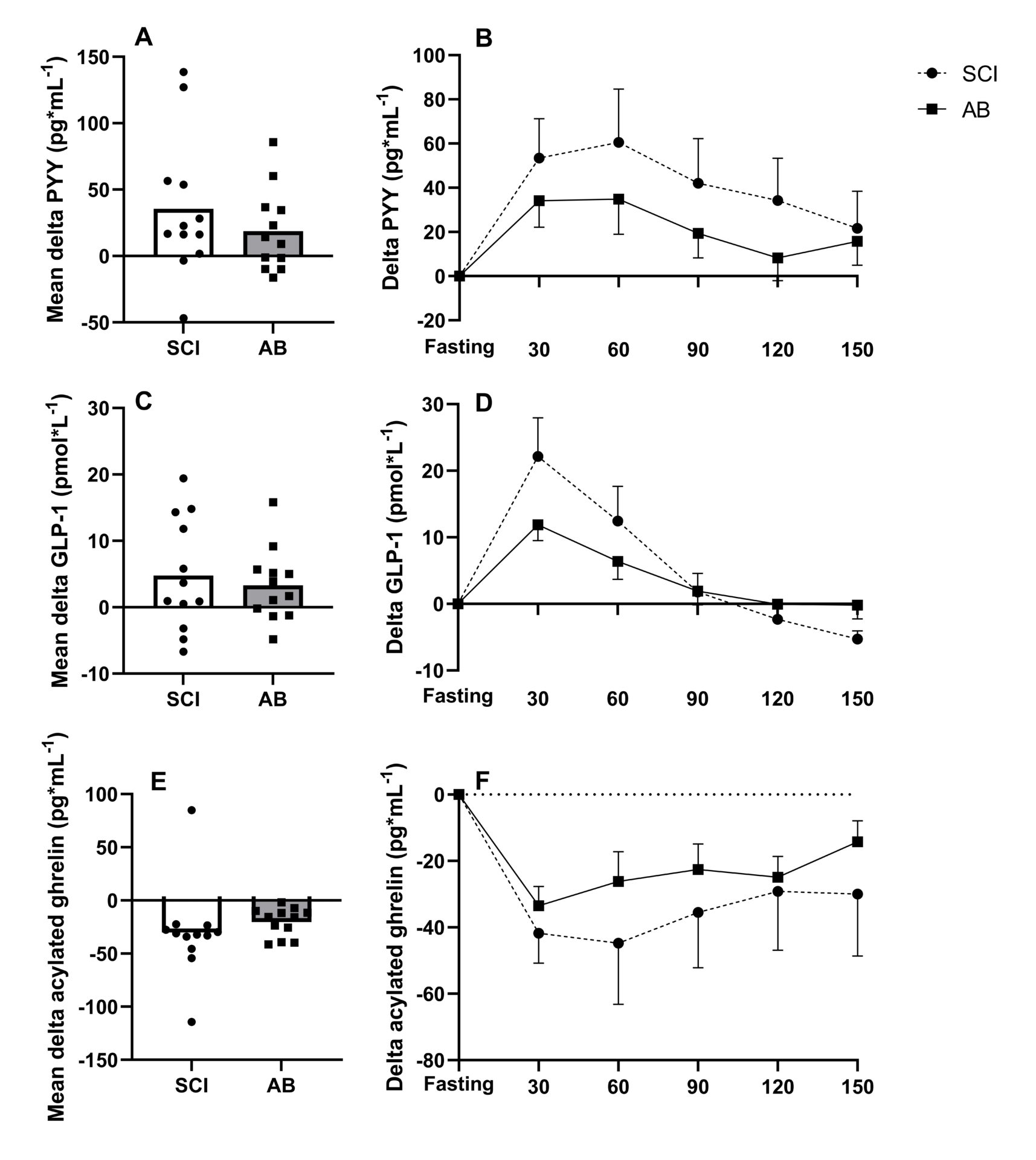
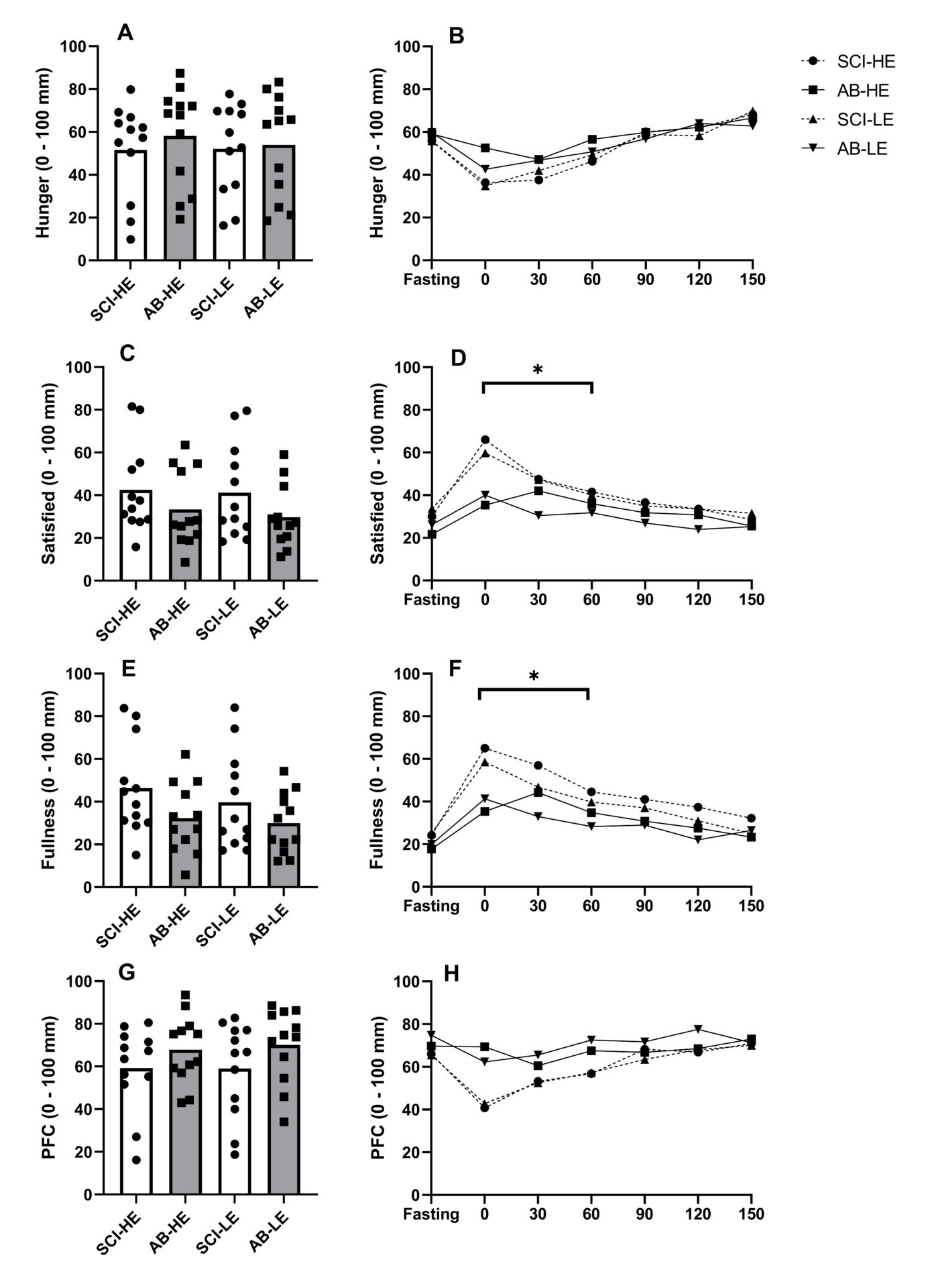
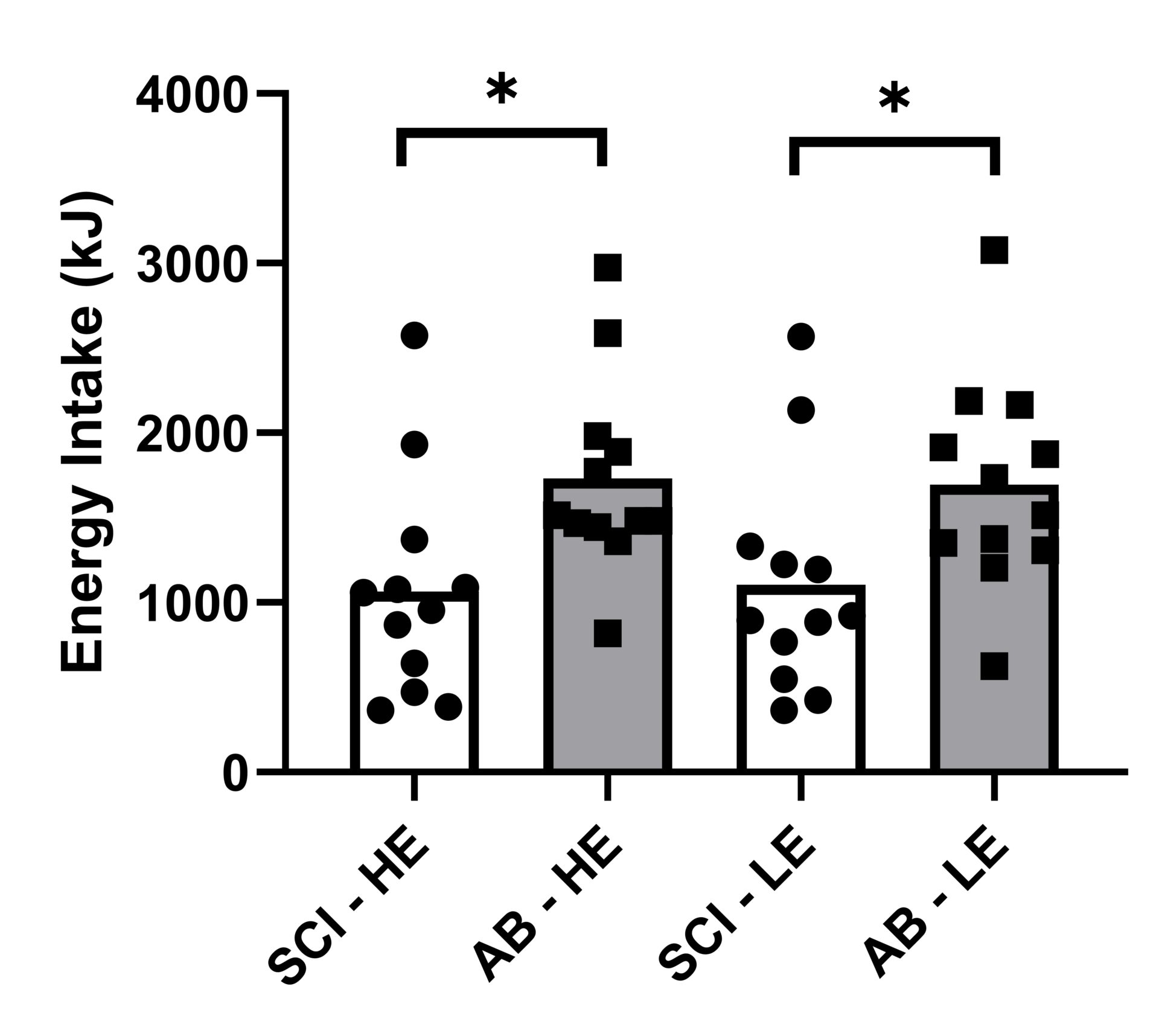
Introduction: In persons with spinal cord injury (SCI), reduced fat-free mass and movement-related energy expenditure contribute to the increased obesity risk found in this population. Impairments in gastrointestinal function in this population are well established (Holmes & Blanke, 2019), but the regulation of appetite-related hormones, and their influence on energy intake regulation remains unknown. Additionally, low levels of total daily energy expenditure have been linked to reduced appetite sensitivity and weight gain (Long et al., 2002), potentially further impacting on energy intake regulation in persons with SCI. This study compared postprandial responses of appetite-related hormones, appetite perceptions and appetite sensitivity in persons with SCI and AB controls. Methods: In a counterbalanced order, 12 men with high-level SCI (≥T4 vertebrae) and 12 body mass and age-matched AB controls completed two trials, consuming a covert high-energy (HE; 2513 kJ) and low-energy (LE; 1008 kJ) preload on separate occasions. Subjective appetite perceptions (hunger, fullness, satisfaction and prospective future consumption [PFC]) were assessed at 30 min intervals following preload consumption (up to 150 min) and appetite sensitivity was subsequently determined from ad libitum meals. Appetite-related hormone (total PYY, GLP-1 and acylated ghrelin) responses were measured in the HE trial. Test-specific effect sizes (Cohen’s d) were calculated in adjunct to each statistical test (a large, moderate, small and trivial effect size was defined as ≥ 0.8, 0.5 < 0.8, and 0.2 < 0.5, and < 0.2, respectively) (Cohen, 1988). Results: Within the early postprandial phase (0-60 min), the SCI group displayed accentuated meal-responses for PYY (d = 0.72; p = 0.365), GLP-1 (d = 1.00; p = 0.134) and acylated ghrelin (d = 0.87; p = 0.112), though no group difference were seen from 60-150 min (d ≤ 0.59; p ≥ 0.340) (Figure 1). Moreover, subjective ratings of fullness (d = 1.09) and satisfaction (d = 0.91) were higher (P ≤ 0.028) during the first hour (Figure 2). Ad libitum energy intake was also lower in the SCI group (1086 vs. 1713 kJ, respectively, d = 1.00; P = 0.020) but no effect of trial (preload) was found (d = 0.003; p = 0.974) (Figure 3). Discussion: These findings suggest that postprandial satiety may be augmented in people with SCI. Such responses may be related to colonic dysfunction, which is believed to reduce motility in the upper gastrointestinal tract (Fynne et al., 2012). As PYY and GLP-1, and AG play a role in the inhibition and stimulation of gastrointestinal motility, respectively, our findings suggest that the accentuated postprandial gut hormone secretion may be mediated via colonic dysfunction. Additionally, reduced lean mass, commonly found in persons with SCI, may be related to the augmented satiety response (Gorgey & Dudley, 2007). In conclusion, this study shows that individuals with SCI exhibit accentuated appetite-related hormone and perceived satiety responses in the early postprandial phase, compared to age and weight-matched AB controls. These findings may reflect wider gut dysmotility in people with SCI but imply that weakened satiety may not be associated with higher rates of obesity.
Future Physiology 2021 (Virutal) (2021) Proc Physiol Soc 47, OC14
Oral Communications: Accentuated early postprandial appetite and appetite-related hormone responses in people with SCI versus able-bodied controls
Jordan Fenton1, James King2, Sven Hoekstra1, Scott Willis2, Takahiro Ogawa3, Vicky Tolfrey1
1 The Peter Harrison Centre for Disability Sport; School of Sport, Exercise and Health Sciences; Loughborough University, Loughborough, United Kingdom 2 Loughborough University, Loughborough, United Kingdom 3 Department of Rehabilitation Medicine, Wakayama Medical University, Wakayama, Japan
View other abstracts by:
Where applicable, experiments conform with Society ethical requirements.