Introduction: Targeting systemic metabolism through the induction of brown adipose tissue (BAT) thermogenesis is an emerging therapeutic strategy for cardiometabolic disease. Activation of BAT thermogenesis reduces key cardiovascular disease risk factors, including weight, ectopic fat accumulation, and dyslipidemia by increasing lipid and glucose oxidation to regulate systemic energy balance. BAT activation occurs through the sympathetic nervous system (SNS), which releases norepinephrine. Norepinephrine binds to beta-adrenergic receptors (beta-ARs) on brown adipocytes, leading to non-shivering thermogenesis in BAT and consequently increased respiration at the whole animal level. The β-site amyloid precursor protein (APP) cleaving enzyme 1 (BACE1) is responsible for the rate-limiting step in the production of β-amyloid (Aβ) peptides and has been implicated in vascular dysfunction in Alzheimer’s disease and more recently in obesity and type 2 diabetes.
Aim: This study aims to understand the involvement of BACE1 in the thermogenesis of BAT and to determine if genetic removal of BACE1 could activate BAT, thereby reducing circulating lipid levels and contributing to weight loss.
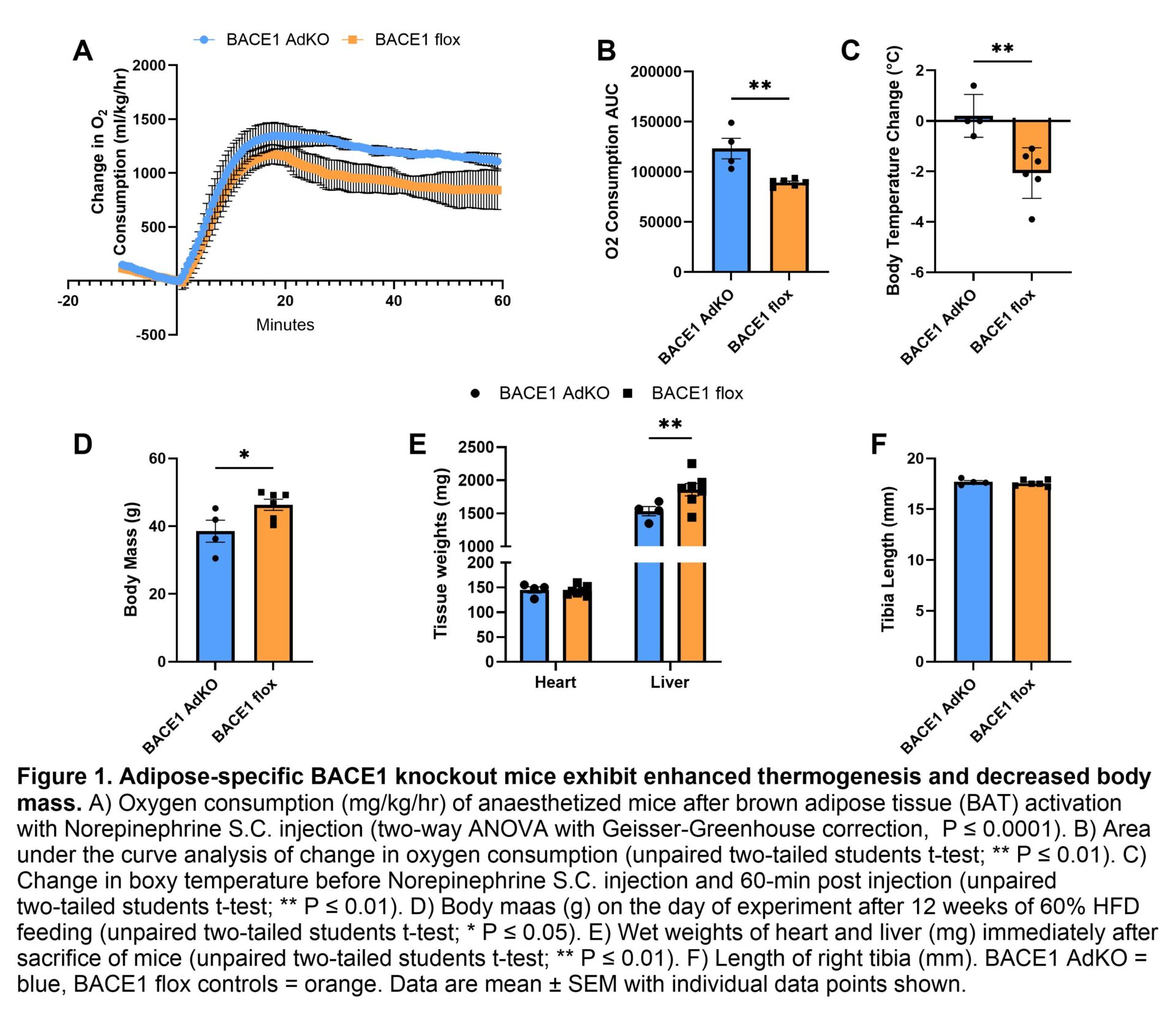
Method: Adipocyte-specific BACE1 knockout mice (BACE1 AdKO) mice were fed a 60% high-fat diet (HFD) for 12 weeks from 8 weeks of age, compared to control mice (BACE1 flox). After 12 weeks of HFD feeding, mice were weighed then anesthetised with a Ketamine/Xylazine/Acepromazine cocktail and placed into the Comprehensive Lab Animal Monitoring System (CLAMS) to measure oxygen consumption (ml/kg/hr) every 30 seconds. After baseline measurements of oxygen consumption, rectal body temperature was measured before mice were injected with norepinephrine (1mg/kg). Oxygen consumption was measured for a further 60 minutes. At the end of the experiment, body temperature was re-measured and mice were sacrificed. Heart and liver weights were recorded, and tissues were frozen for further analysis.
Results: We identified that specifically knocking out BACE1 in adipose tissue increases oxygen consumption in mice after stimulation with norepinephrine, indicating enhanced BAT thermogenic capacity. The area under the curve (AUC) for oxygen consumption was significantly higher in BACE1 AdKO (n = 4) compared to control mice (BACE1 flox n = 6) (Figure 1A & B). Adipose-specific BACE1 knockout mice maintained body temperature throughout the experiment, with an average increase of 0.2°C, whereas control mice showed an average decrease of 2.1°C (Figure 1C). BACE1 AdKO mice exhibited a 16.8% decrease in body mass compared to control mice (Figure 1D), with significantly reduced liver mass but no significant difference in heart mass between groups (Figure 1E). The change in body mass was not due to differences in mouse size, as tibia length remained unchanged between groups (Figure 1F).
Conclusions: Knocking out BACE1 in adipose tissue results in reduced weight gain and increased norepinephrine-stimulated whole-body respiration, identifying BACE1 as a negative regulator of BAT thermogenesis. Further research will continue to explore BACE1’s role within adipose tissue, hypothesising that BACE1 inhibition enhances brown adipose thermogenesis.