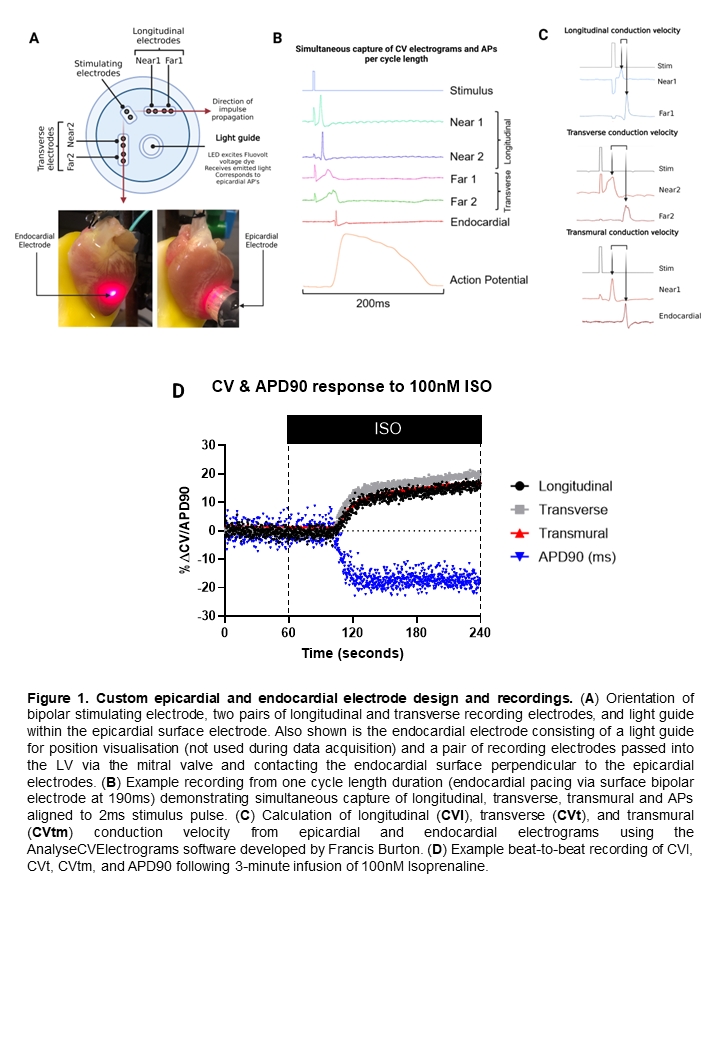
Introduction – Conduction velocity (CV) is an important determinant of the arrhythmic status of the ventricle. SNS-mediated changes in CV may contribute to the normal ventricular activation sequence. We utilised a custom-made electrode array to record epicardial electrograms at sites in longitudinal, transverse, and transmural directions that incorporates a light guide to allow optical AP measurements from the electrogram recording area (Fig. 1). This set-up allows beat-to-beat assessment of myocyte to myocyte conduction and electrophysiological parameters to examine the magnitude and time course of pharmacologically mediated changes targeting the adrenergic signalling pathway. It has been well established that β-adrenoceptor expression and sympathetic nerve remodelling are pathological features of heart failure. Our approach will resolve CV changes in response to adrenergic modulation initially in the healthy heart which will then form the basis of our comparison to adrenergic signalling post-MI.
Methods – The hearts of adult, male New Zealand White rabbits were rapidly excised following terminal Euthatal administration via marginal ear vein according to UK Home Office approval and legislation. Hearts were Langendorff-perfused at 37°C and perfused with the voltage sensitive dye FluoVoltTM to record APs. For transmural recordings, an electrode was then inserted via the left atria into the LV and placed against the endocardial surface of the mid LV free wall at a corresponding point to the endocardial electrode to measure transmural conduction velocity (CVtm). The custom endocardial electrode comprised of a pair of stimulating electrodes and two pairs of recording electrodes at a 90o angle from each other corresponding to the longitudinal (CVl) and transverse (CVt) epicardial fibre orientation. Each CVl and CVt recording electrodes were spaced at a 2mm distance and used to calculate epicardial conduction velocity. To calculate CVtm, the thickness of the LV mid-wall was determined post-experiment.
Results – The high sampling rates (20kHz) enabled us to document small electrophysiological changes including alternans in both AP and CV measurements. Thus far, we have resolved CV and APD changes as small as 1%. 100nM Isoprenaline elicits an increase in CVl (13.2%), CVt (14.4%), and CVtm (12.8%) with a corresponding decrease in APD90 of -16.5% (n=8). Interestingly, increasing intracellular Ca2+, a known effect of SNS activation, via infusion of 5mM Ca2+ resulted in a reduction in CVl (-13.0%), CVt (-11.0%), and CVtm (-9.0%) with a corresponding decrease in APD90 of -9.1% (n=5). Mimicking SNS activation by raising increasing intracellular cAMP via 15µM Forskolin and 50µM IBMX, a similar pattern emerged of CV and APD changes as Isoprenaline in CVl (11.1%), CVt (12.1%), CVtm (10.66%), and APD90 (-13.20%) (n=8). These findings also suggest that the increase in CV brought about by adrenergic stimulation must also overcome the reduction in CV which is a result of increased intracellular Ca2+.
Conclusions – By utilising this novel electrode and light guide system, we have begun to establish the effect of adrenergic signaling on the interplay between increased myocyte to myocyte conduction velocity and action potential characteristics at a resolution not previously achieved.