BACKGROUND: SARS-CoV-2 is the virus responsible for the ongoing COVID-19 pandemic. Although this virus affects people of all ages, studies have shown that the elderly are at a higher risk of severe disease and death from COVID-19 compared to children, who once infected with SARS-CoV-2 rarely progress to respiratory failure. We aimed to investigate this by studding how the cells lining the nose respond to SARS-CoV-2 infection in people of different ages.
METHODS: To do this, we cultured differentiated primary nasal epithelial cells (NECs) at air-liquid interface from three different age groups: paediatric (<14 years, n=11), adult (30-50 years, n=9), and elderly (>70 years, n=9) individuals. Ethical approval was given through the Living Airway Biobank (REC reference: 19/NW/0171). We then used a comprehensive, multidisciplinary approach using functional assays and scRNAseq to analyse the cellular landscape of the infected cultures and examined the replication of the virus within the different cell subtypes.
|
Study Population |
Total cultures analysed (n) |
Total cells for scRNAseq |
||
|
Total n |
29 |
|
251 |
|
|
% Female |
41% |
|
38% |
|
|
|
|
|
|
|
|
Brushings |
n |
Age (mean ±SD) |
n |
|
|
Paediatric (0-11y) |
14 |
4.9 ±4.2​ |
118 |
32,892 |
|
Adult (30-50y) |
9 |
36.9 ±2.7​ |
65 |
​56,221 |
|
Elderly (70y+) |
9 |
83.6 ±6.7​ |
68 |
​50,485 |
|
|
|
|
Total cells |
139,598 |
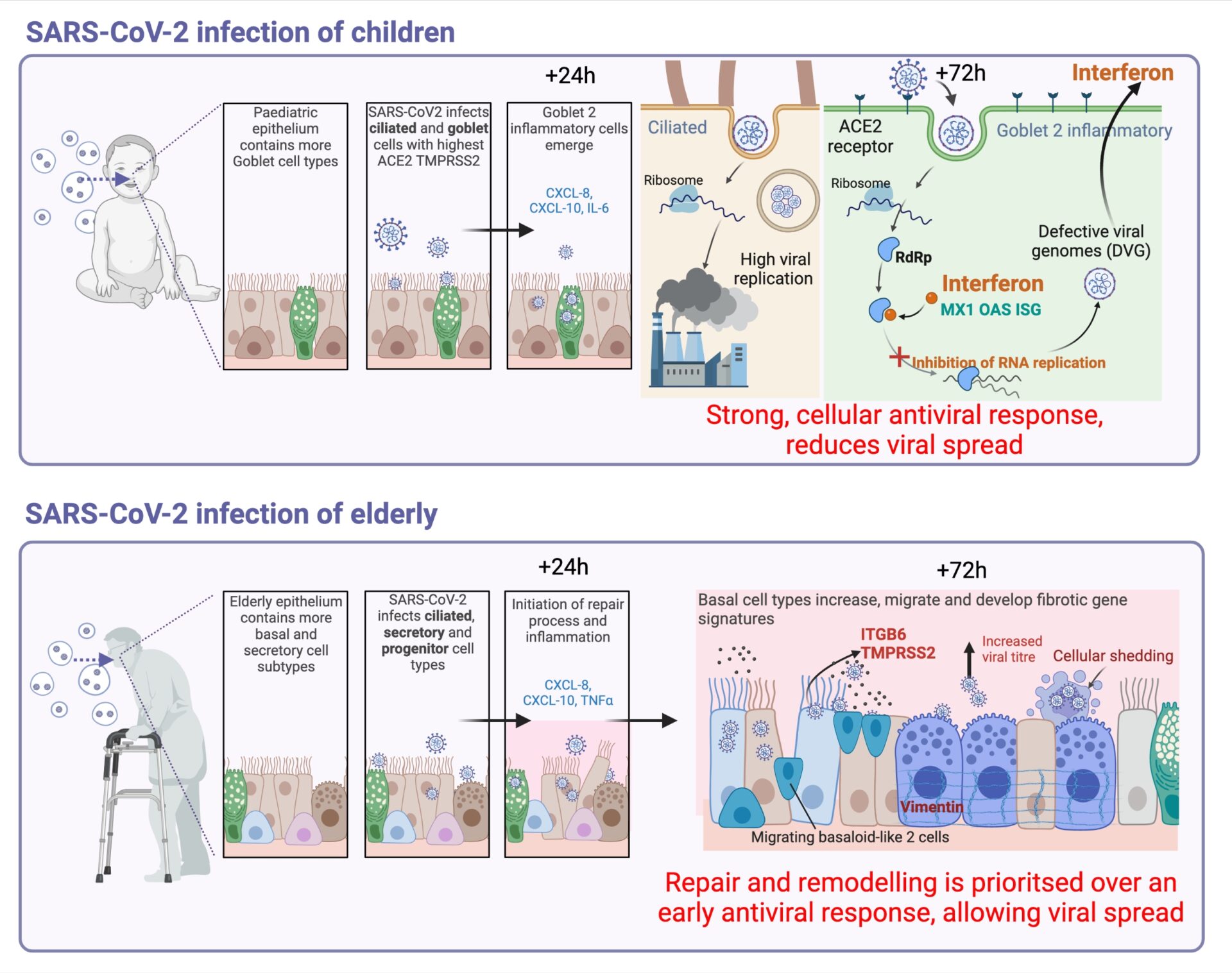
RESULTS: Our data revealed that nasal epithelial cell subtypes show different tropism to SARS-CoV-2, correlating with age and ACE2 and TMPRSS2 expression. For example, we found that ciliated cells are a viral replication centre across all age groups, but a distinct goblet inflammatory subtype emerges in infected paediatric cultures, identifiable by high expression of interferon-stimulated genes, truncated viral genomes, greater sub-genomic viral RNA, and less infectious progeny compared to older adult cultures. On the other hand, SARS-CoV-2 infected elderly secretory cells were shed, and cultures suffered greater epithelial damage with age. Dysfunctional repair pathways were stimulated, and there was an increase in basaloid-like cells that are associated with fibrosis markers and greater viral spread. We hypothesized that SARS-CoV-2 infected nasal epithelial cells undergo reprogramming by these mechanisms in an age-dependent manner and that these processes contribute to COVID-19 pathogenesis by delaying disease resolution and enhancing viral spread.
CONCLUSIONS: Our study provides new insights into age-associated COVID-19 pathogenesis. We found that SARS-CoV-2 exhibits differential tropism for nasal epithelial cells with age, with preferential infection of paediatric goblet or elderly secretory cell types. Infected paediatric goblet cells mount a robust innate antiviral response to SARS-CoV-2 dominated by interferon, which correlates with reduction in infectious viral load. In the elderly dysfunctional repair pathways are stimulated, and there is an increase in basaloid-like cells that are associated with fibrosis markers and greater viral spread. These insights could aid in the development of new treatments for COVID-19, particularly for older individuals who are at greater risk of severe infection.
This work is currently published as a preprint: https://www.biorxiv.org/content/10.1101/2023.01.16.524211v2.full