Background: Ageing results in a gradual loss of skeletal muscle mass and type II fibre atrophy, through dysregulated muscle protein synthesis (MPS). Traditional immunoblotting techniques have revealed dysregulated mTOR-mediated signalling as a mechanistic driver of impaired MPS in older adults. However, immunoblotting cannot determine fibre-type specific protein abundance, localisation, or translocation of these molecular regulators within cells. Immunofluorescence microscopy (IF) has the capacity to address the shortcomings associated with immunoblotting and uncover mechanisms of age-related MPS impairment and muscle decline. This study aimed to use IF to measure age-related and fibre-specific differences in the abundance and localisation of mTOR-mediated proteins.
Methods: Resting muscle biopsies from eight young males (YM; 24 ± 4 years, BMI; 24 ± 4 kg·m2) and seven older males (OM; 67 ± 5 years, BMI; 25 ± 2 kg·m2) were embedded in OCT compound, frozen in liquid nitrogen-cooled isopentane for IF subsequent analysis. Embedded muscle samples were cut using a microtome blade, and cryosections (7mm) were collected on room-temperature uncoated glass slides. Sections were fixed and incubated in MHC-I, Rheb, TSC2 or WGA, mTOR and Sestrin-2 primary antibodies and incubated in contrasting Alexa Fluor secondary antibodies. Images were captured at 10x for fibre type distribution and 20x for analysis of fibre-type immunofluorescent abundance. Full ethical approval was granted (18/EM/0004) and procedures were conducted in accordance with the Declaration of Helsinki.
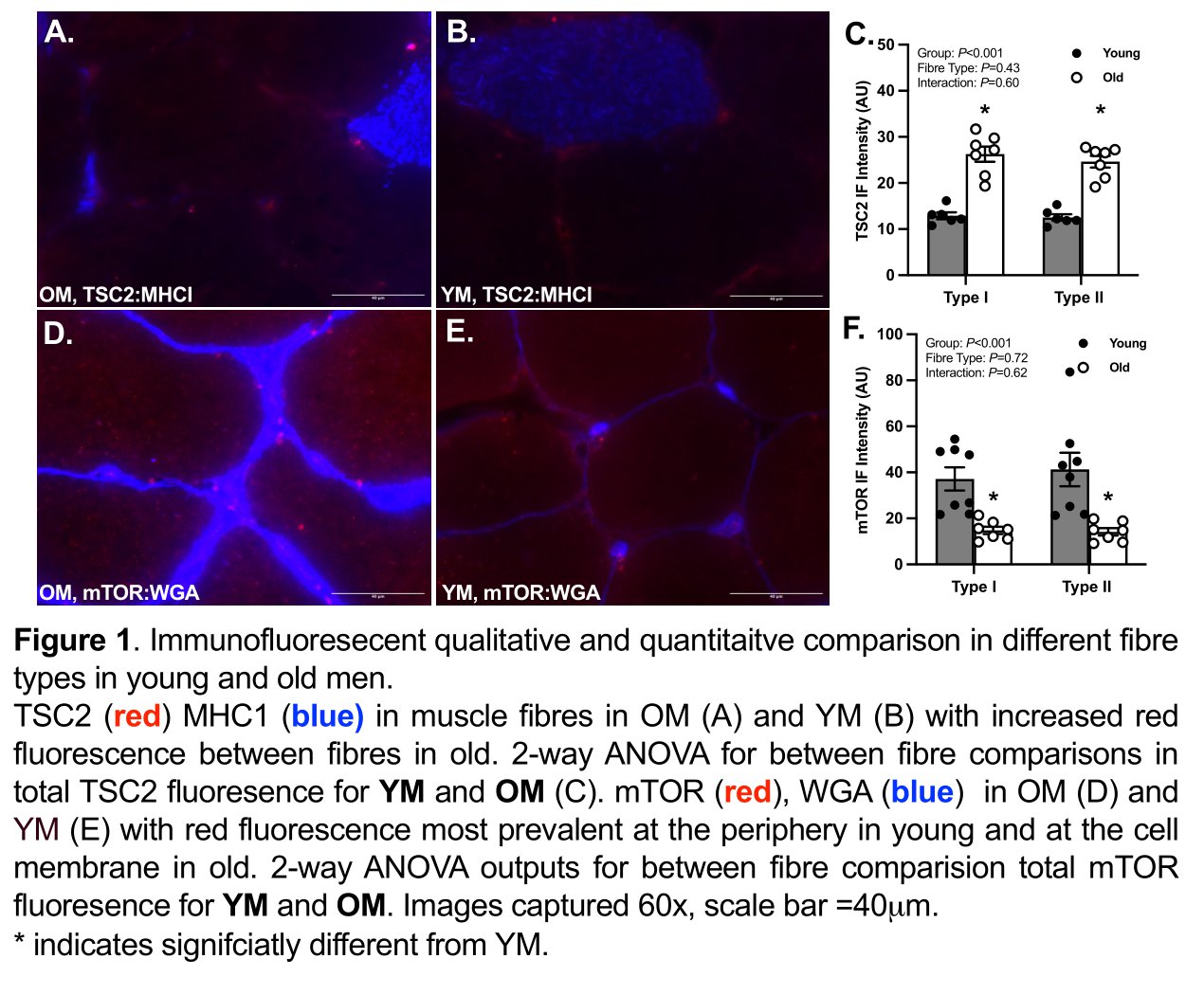
Results: OM displayed a significantly (P=0.0151) lower proportion of type I fibres than YM (YM, 57.6 ± 0.6% vs OM, 47.6 ± 2.8%). Mean CSA was not different between groups for type I (YM, 4745.8 ± 98.1 mm2 vs OM, 6133.0 ± 408.6 mm2) or type II fibres (YM, 6556.7 ± 196.0 mm2 vs OM, 5752.3 ± 246.8 mm2). Notably, a 2-fold higher abundance of TSC2 in OM compared with YM (YM, 12.71 ± 0.28 A.U. vs OM, 25.45 ± 0.64 A.U.; P<0.001) was observed. OM displayed a ~31% (YM, 68.86 ± 3.36 A.U. vs OM, 47.22 ± 2.77 A.U., P=0.020) and a ~63% (YM, 39.21 ± 2.15 A.U. vs OM, 14.49 ± 0.57 A.U., P<0.001) lower abundance in Sestrin-2 and mTOR, respectively, compared with YM. Rheb abundance was ~43% higher in OM compared to YM (YM, 18.66 ± 0.92 A.U. vs OM, 26.71 ± 0.58 A.U., P<0.001). There were no differences in protein target abundance between fibre types for either group. Notably, peripheral and membrane bound fluorescence was higher in OM.
Discussion: Using IF, it is possible to characterise fibre-specific molecular regulators of skeletal muscle. Whilst the selection of regulatory proteins we investigated were similarly abundant in type I and II fibres, differences in mean fluorescence relative to fibre area of mTOR, TSC2, Sestrin-2 and Rheb may be implicated in age-related MPS impairment and muscle decline. IF could be used to identify dysregulated signalling events that underpin age-related anabolic resistance (e.g., impaired MPS response to amino acids and/or contraction).
Conclusion: IF characterisation of the fibre-type-specific abundance of key regulators of mTORC1 revealed age-related differences that may be linked to dysregulated proteostasis.