The Kir3.1-Kir3.4 channel is activated by Gβλ subunits released on binding of acetylcholine to the m2 muscarinic receptor. A mechanism of channel opening has been suggested that involves translocation of pore lining transmembrane helices and the opening of an intracellular gate (Jin et al. 2002). Here we show that an extracellular gate at the selectivity filter is critical for agonist activation of the Kir3.1-Kir3.4 channel.
Kir3.1, Kir3.4 and the dopamine D2 receptor (another G protein-coupled receptor) were injected into Xenopus oocytes and currents were recorded 24-96 h later using the two-electrode voltage clamp technique; 750 ms voltage pulses were applied to -130 to +60 mV from 0 mV in the presence of 90 mM K+.
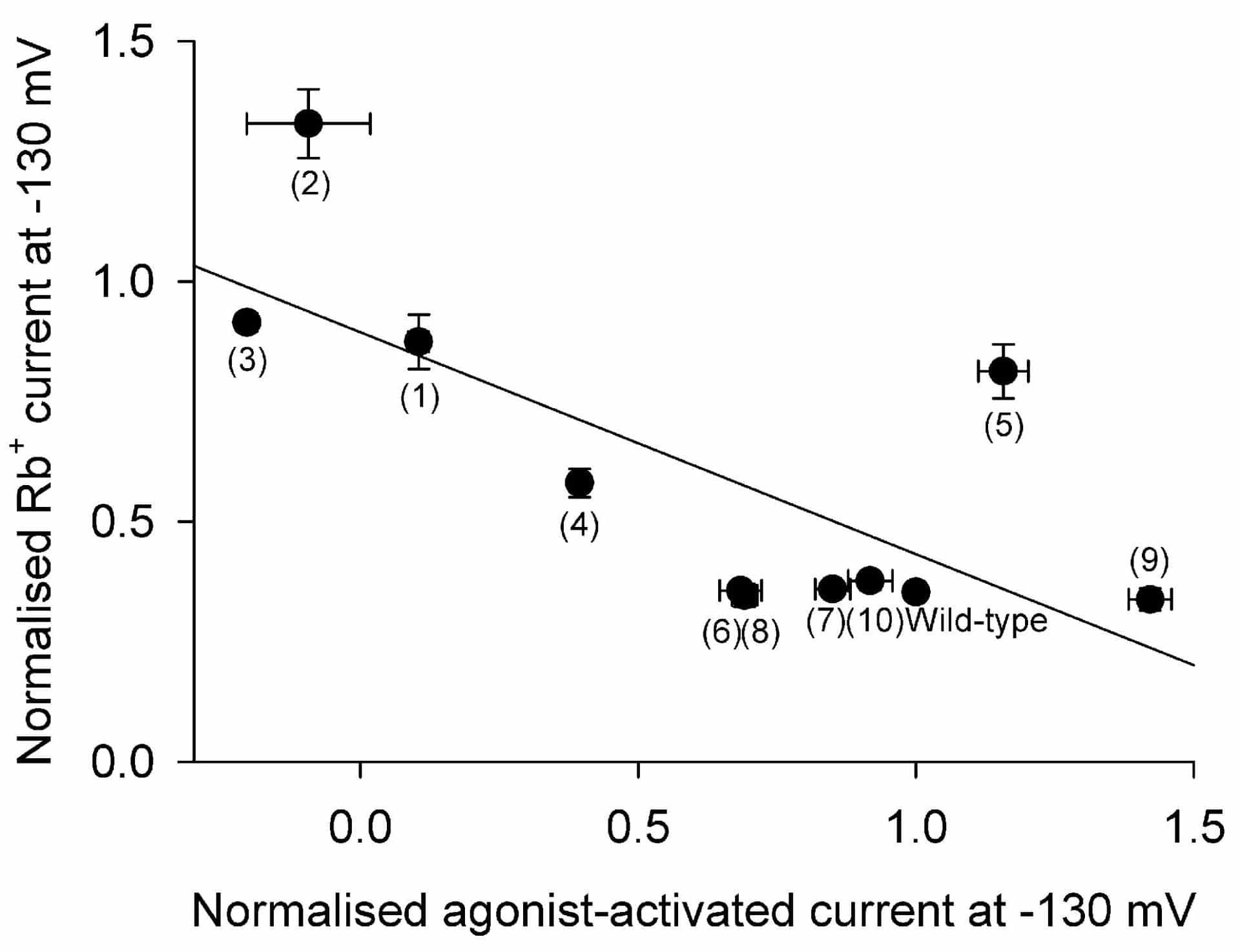
Current was seen in the absence of agonist, but on application of 10 µM dopamine wild-type Kir3.1-Kir3.4 current was increased by 60.7 ± 6.4 % (mean ± S.E.M., n = 23). Figure 1 shows evidence that the selectivity filter may be involved in agonist activation: the extent of agonist activation (agonist-activated current at -130 mV normalised to that in the wild-type channel) is plotted against a measure of channel selectivity (current at -130 mV with Rb+ as the charge carrier normalised to that with K+ as the charge carrier). There is a significant correlation (r2 = 0.52; Pearson coefficient, P < 0.02) between agonist activation and selectivity. Mutations that abolished selectivity, such as those that disrupted a salt bridge that maintains the selectivity filter structure (Kir3.4-E145Q (1), Kir3.4-R155E (2) or Kir3.4-E145R, R155E (3)), also abolished sensitivity to agonist. Another mutation Kir3.4-T148A (4) that is important for extracellular block and permeation (Makary et al. 2003) halved the effect of agonist and doubled the size of the Rb+ current. Other mutations within the selectivity filter, such as Kir3.1-E139Q (5) (equivalent to Kir3.4-E145Q), Kir3.1-A142T (6) (equivalent to Kir3.4-T148A) or the double mutation Kir3.1-A142T/Kir3.4-T148A (7), that had little or no effect on selectivity also had little or no effect on agonist-activation. Finally, mutations at the intracellular gate (Kir3.1-F181M (8), Kir3.4-F187M (9) and Kir3.1-F181M-Kir3.4-F187M (10)) had no effect on selectivity and only a small effect on agonist activation.
These results demonstrate a significant correlation between agonist activation and selectivity, which is determined by the selectivity filter, and suggests, therefore, that agonist activation of the Kir3.1-Kir3.4 channel may involve the selectivity filter.