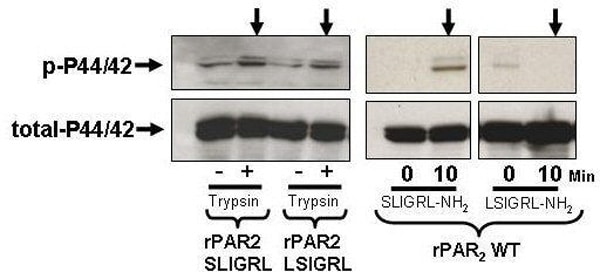
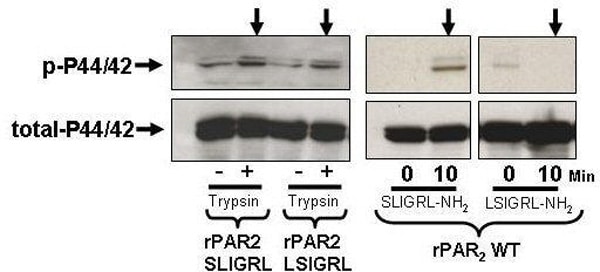
Proteinase activated receptors (PARs) are a family of GPCR’s that are activated by serine proteinase cleavage of the receptor N-terminal sequence to reveal a tethered receptor-activating ligand (t.l.) (e.g. SLIGRLDTP….for rat PAR2). Our previous work (Mol. Pharm. 65:149, 2004) employing alanine replacements of the first six amino acids of the trypsin-revealed t.l. of rat (r) PAR2, showed that the t.l. sequences, SLIGRL… and SLAAAA… were able to generate a calcium signal, whereas the t.l. sequences, AALIGRL.. and LSIGRL… did not. Further, the synthetic peptides SLAAAA-amide, AALIGRL-amide and LSIGRL-amide were not active. We hypothesized that, while unable to cause a calcium signal, trypsin-revealed t.l. sequences like AAIGRL… and LSIGRL… might still be able to signal via MAPkinase activation. We utilized Kirsten virus-transformed rat kidney cells transfected with wild-type rPAR2 and rPAR2 mutants with N-terminal tethered ligand alanine substitution modifications, to generate receptors with modified t.l. sequences: rPAR2,LSIGRL, rPAR2,SLAAAA and rPAR2,AAIGRL. All receptors were treated with 10nM trypsin to unmask the t.l. sequences and both calcium signaling and P42/44 MAPkinase activation at 10 min. were monitored by fluo-3 fluorescence and western blot analysis. Receptor-expressing cells were similarly exposed to synthetic peptides (SLIGRL-NH2, LSIGRL-NH2, SLAAAA-NH2 and AAIGRL-NH2; 10μM) corresponding to the wild-type and modified N-terminal t.l. sequences. All trypsin-revealed t.l. sequences caused MAPkinase activation, but only the t.l. sequences SLIGRL… and SLAAAA… triggered a significant calcium signal. All peptides, except for SLIGRL-NH2, failed to signal via either calcium or MAPkinase. We conclude that different trypsin-revealed rat PAR2 t.l. sequences can trigger selective signal trafficking by either calcium transients or the activation of MAPkinase, presumably by promoting interactions selectively with either Gq or Gi. Further our data demonstrate dramatic differences in both calcium and MAPkinase signaling by the PAR2 activating sequences either as trypsin-revealed t.l.s or as synthetic receptor-activating peptides.
Life Sciences 2007 (2007) Proc Life Sciences, PC470
Poster Communications: Agonist-mediated signal trafficking by Proteinase-activated receptor-2 (PAR2): Differential activation of calcium and MAPkinase signaling by distinct trypsin-revealed tethered ligand sequences
R. Ramachandran1, M. D. Hollenberg1
1. Pharmacology and Therapeutics, University of Calgary, Calgary, AB, Canada.
View other abstracts by:
Differential MAPK signaling by trypsin-revealed TL sequences and synthetic PAR-activating peptides.
Table 1: rPAR2 constructs with their proteolytically revealed tethered ligand and synthetic activating peptides.
Where applicable, experiments conform with Society ethical requirements.