It has been shown that the 5fiAMP activated protein kinase (AMPK) purified from rat liver phosphorylates glycogen synthase (GS) at serine N7 in vitro (Carling & Hardie, 1989). Phosphorylation of N7 under in vivo conditions reduces GS activity in skeletal muscle (Poulter et al. 1988). Therefore, it has been suggested that AMPK phosphorylates and deactivates GS in vivo. It is also well established that GS activity is inhibited by increased muscle glycogen content (Danforth, 1965). The aim of the present study was to elucidate whether AMPK stimulated with 5-aminoimidazole-4-carboxamide ribonucleoside (AICAR) causes a GS deactivation in rat skeletal muscles in a glycogen-dependent manner.
Rat hindlimbs were perfused with either vehicle (Krebs Henseleit Ringer buffer) or 2 mM AICAR. The muscle glycogen content was manipulated the day before perfusion to either high (HG) or low glycogen level (LG) by a swimming and diet procedure. Muscle biopsies were taken from soleus and the white part of gastrocnemius (WG), and activities of GS and AMPK α-isoforms as well as content of adenosine nucleotides were measured. Animals were anaesthetised with pentobarbital I.P. (5 mg (100 g)-1) and humanely killed by cervical dislocation. Experiments were carried out according to European and Danish legislation.
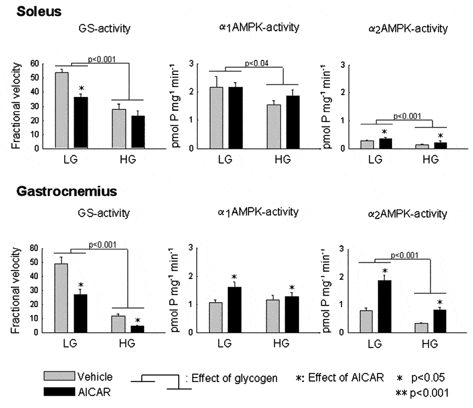
AICAR induced a 35-50 % decrease in GS activity primarily in the LG group independent of muscle type (P < 0.05), whereas GS activity in the HG group was reduced only in WG (P < 0.05) (Fig. 1). The deactivation of GS was accompanied by a decreased gel mobility, which could be eliminated by protein phosphatase treatment, indicating that change in GS activity was due to phosphorylation. AICAR induced an increase in activity of both the α2AMPK and α1AMPK isoforms. Glycogen loading induced a ~50 % reduction in α2 AMPK-activity in both muscles, whereas the α1 AMPK-activity was influenced to a minor degree (Fig. 1). In WG, but not in soleus, α2 AMPK-activity correlated negatively (r 2 = 0.56) with GS activity. The level of adenosine nucleotides was not affected by AICAR-treatment.
AICAR induced a phosphorylation-dependent deactivation of GS in both muscles most pronounced in the LG group. Since AICAR activates AMPK, and because AMPK phosphorylates GS in vitro, it is likely that AMPK is responsible for the in vivo deactivation of GS. This is partly supported by the negative correlation found between GS and α2AMPK activity in WG. However, the mobility shift of GS was greater than expected if induced by only a single N7 GS phosphorylation. This could indicate that more than one site on GS was phosphorylated by an unknown AICAR sensitive protein kinase. To conclude, AICAR induced a phosphorylation-dependent deactivation of GS, which may be explained by the increased α2 AMPK activity.
 View larger image [new window] |
| Figure 1. Fractional velocity of glycogen synthase (GS) and kinase activity of both α-isoforms of AMPK in soleus and white part of gastrocnemius muscle preconditioned to either high (HG) or low (LG) glycogen content. Statistical significance (n = 6-8) was tested with 2-way ANOVA, and variation expressed as standard error. |