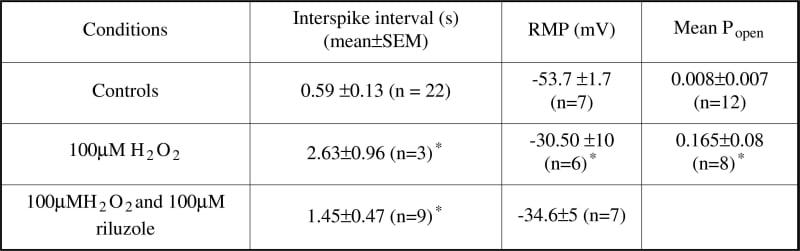
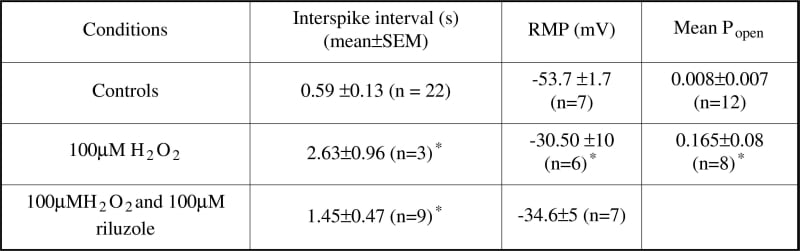
Oxidative stress (OS) is implicated in Amyotrophic Lateral Sclerosis (ALS), a progressive and fatal neurodegenerative disease (Cookson and Shaw, 1999). The present study was designed to assess the functional changes in motor neurones (MNs) following exposure to OS using cell-attached patches to measure the effects of H2O2 on both cellular excitability and single channel activity. Spinal cord cultures were grown from humanely killed E13 mice (Boolaky et al. 2002), MNs being identified morphologically and immunohistochemically. Whole-cell and cell-attached recordings were made. The bath solution contained (mM): 140 NaCl; 3 KCl; 2 MgCl2; 2 CaCl2; 10 HEPES; 10 Glucose, pH 7.4. The pipette solution contained (mM): 140 KCl; 5 NaCl; 1 MgCl2; 1 CaCl2; EGTA 11; HEPES 10, pH 7.2. In single-channel experiments 1 μM TTX was added to the bath solution. Cells were patched under control conditions and following incubation for 1 hour (5% CO2, 37oC) in medium containing 100μM H2O2. All recordings were made at 0mV pipette potential (pp). Electron paramagnetic resonance (EPR) spectroscopic detection of α-phenyl-tert-butylnitrone adducts was incorporated for direct detection of free radicals. Control cells possessed healthy RMP, and 88 % of cells had spontaneous biphasic action currents. Following treatment with 100μM H2O2, both the RMP (Table 1) and the number of cells firing decreased to 59%; the interspike interval, a measure of firing frequency, increased. The Popen of large conductance maxi-K channels, at 0mV pp, was significantly increased in MN (Table 1) and the voltage dependence was decreased following H2O2-treatment (n = 4). Incubation with 100μM riluzole did not prevent membrane depolarisation or the reduction in the firing frequency, although it did prevent reduction in the number of cells firing. EPR spectroscopy showed that incubation with 100μM H2O2 increased the concentration of lipid-derived free radicals identified as a combination of alkoxyl, alkyl and hydroxyl radical species. However, this was not affected following addition of 100μM riluzole. This study demonstrates that OS induced by H2O2 produces significant changes in the excitability of MN in culture. Such changes may mirror the excitability increases in ALS and underpin neurodegeneration
King's College London (2005) J Physiol 565P, PC59
Communications: Altered redox-status modifies excitability in mouse cultured spinal cord neurones
Boolaky, Usha Vicki; Bailey, Damian M; Wann, Kenneth Taylor;
1. Welsh School of Pharmacy, Cardiff University, Cardiff, South Glamorgan, United Kingdom. 2. Departments of Anesthesiology and Surgery, University of Colorado Health Sciences Center, Denver, CO, USA.
View other abstracts by:
Table 1: H2O2 and MN excitability.*P ≤ 0.05 (independent Student's t test).
Table 1: H2O2 and MN excitability.*P ≤ 0.05 (independent Student's t test).
Where applicable, experiments conform with Society ethical requirements.