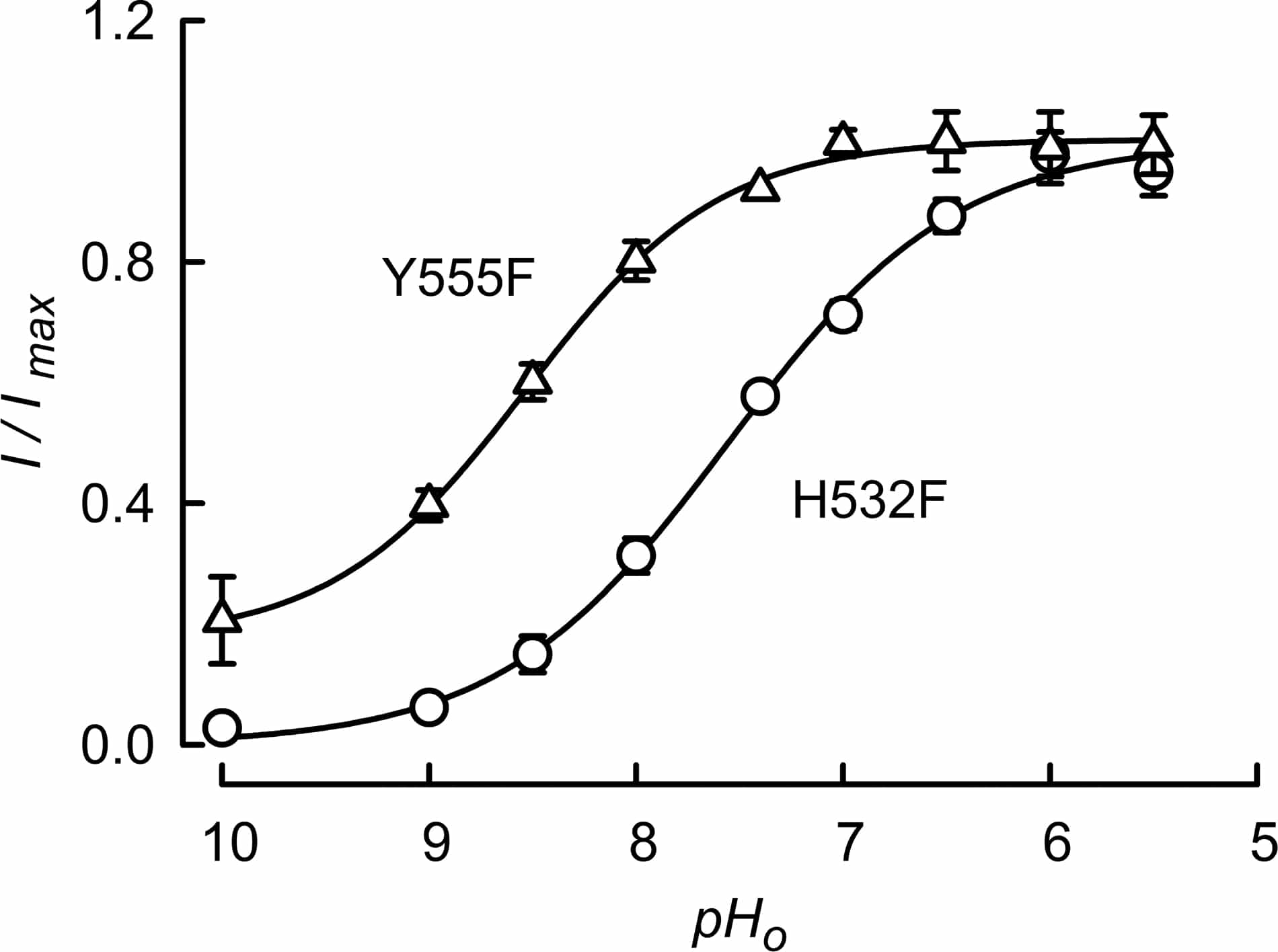
The activity of the ClC-2 chloride channel has a biphasic response to extracellular pH, with activation by moderate acidification followed by abrupt channel closure at pH values lower than ~7. We recently identified H532 as the sensor coupling extracellular acidification to complete closure of the channel (Niemeyer et al., 2009). This extracellularly-facing histidine is located at the N-terminus of transmembrane helix Q that, according to a homology molecular model of the channel, connects via a short loop with Y555, a conserved residue that has been suggested to take part in the gating of certain ClC proteins at an intracellular gate located at the cytoplasmic end of the pore (Bell et al., 2006; Jayaram et al., 2008). We have now investigated whether amino acids in the environment of H532 are important in the acidification-induced channel closure and whether the H532 sensing function depends upon inner pore residue Y555. The homology model for ClC-2 suggests that extracellular loop I-J is in the vicinity of H532. Several charged residues in I-J were neutralised without any effect upon the inhibition of ClC-2 by acidification (mutants examined E302V, E301Q, R311Q and D315N). Three aromatic residues of loop I-J, however, had a marked effect upon acidification-induced inhibition of ClC-2. These were F308, F312 and F316. Double mutants F308A-F312A and F308A-F316A were completely indifferent to acidification. As shown in Figure 1, when Y555 was mutated to F there was a complete disappearance of ClC-2 inhibition by acidification. Our present data highlight the importance of extracellular residues of the I-J loop F308, F312 y F316. Given the closeness of H532 to these residues and their chemical nature, it is tempting to speculate that some aromatic-aromatic, perhaps of the π-π type, interaction occurs between H532 and the mentioned F residues. Protonation of H532 might lead to the disruption of this putative network of interactions and to channel closure. Finally our result showing that ClC-2-Y555F fails to be blocked by extracellular acidification suggests that the effect of H532 charge change might be exerted through a long-range action on a putative intracellular gating mechanism located near the intracellular pore entrance.
University College Dublin (2009) Proc Physiol Soc 15, C61
Oral Communications: An extracellular-facing sensing histidine closes the ClC-2 chloride channel through a long-range effect on the pore
Y. R. Yusef1, R. Briones1, L. Cid1, M. I. Niemeyer1, F. V. Sepúlveda1,2
1. Centro de Estudios Científicos (CECS), Valdivia, Chile. 2. CIN, Centro de Ingeniería de la Innovación asociado al CECS, Valdivia, Chile.
View other abstracts by:
Fig. 1. Effect of extracellular pH on Y555F mutant of ClC-2. The data (triangles) are are averages with SEMs, n=3. The intra- and extracellular solutions contained 35 and 140 mM chloride respectively. The potential used was -130 mV. Data for ClC-2-H532F are from Niemeyer et al. (2009) and are shown for comparison.
Where applicable, experiments conform with Society ethical requirements.