It has been proposed that extracellular neuritic plaques formed by amyloid β peptide (Aβ) deposits may contribute to degenerative changes found in Alzheimer’s disease (AD). Neuroprotective effects of 17β-oestradiol (E2) have been described in cellular and animal models of injury that partially mimic the neurotoxic events of AD. However, the mechanisms underlying oestrogen-related neuroprotection remain unclear. We have shown previously (Guerra et al. 2001) that the 1-40 fragment of human Aβ induces massive cell death in a murine cholinergic cell line (SN56) which expresses functional oestrogen receptors (ERs) (Martin et al. 2001; Martinez-Morales et al. 2001). This effect is prevented by E2 in a dose-dependent manner through a mechanism mediated by ER. We now report that these effects are also observed after short exposures to E2 or the impermeant conjugate oestradiol-peroxidase (E-HRP) and are partially dependent on an ICI 182,780-sensitive ER associated with the plasma membrane.
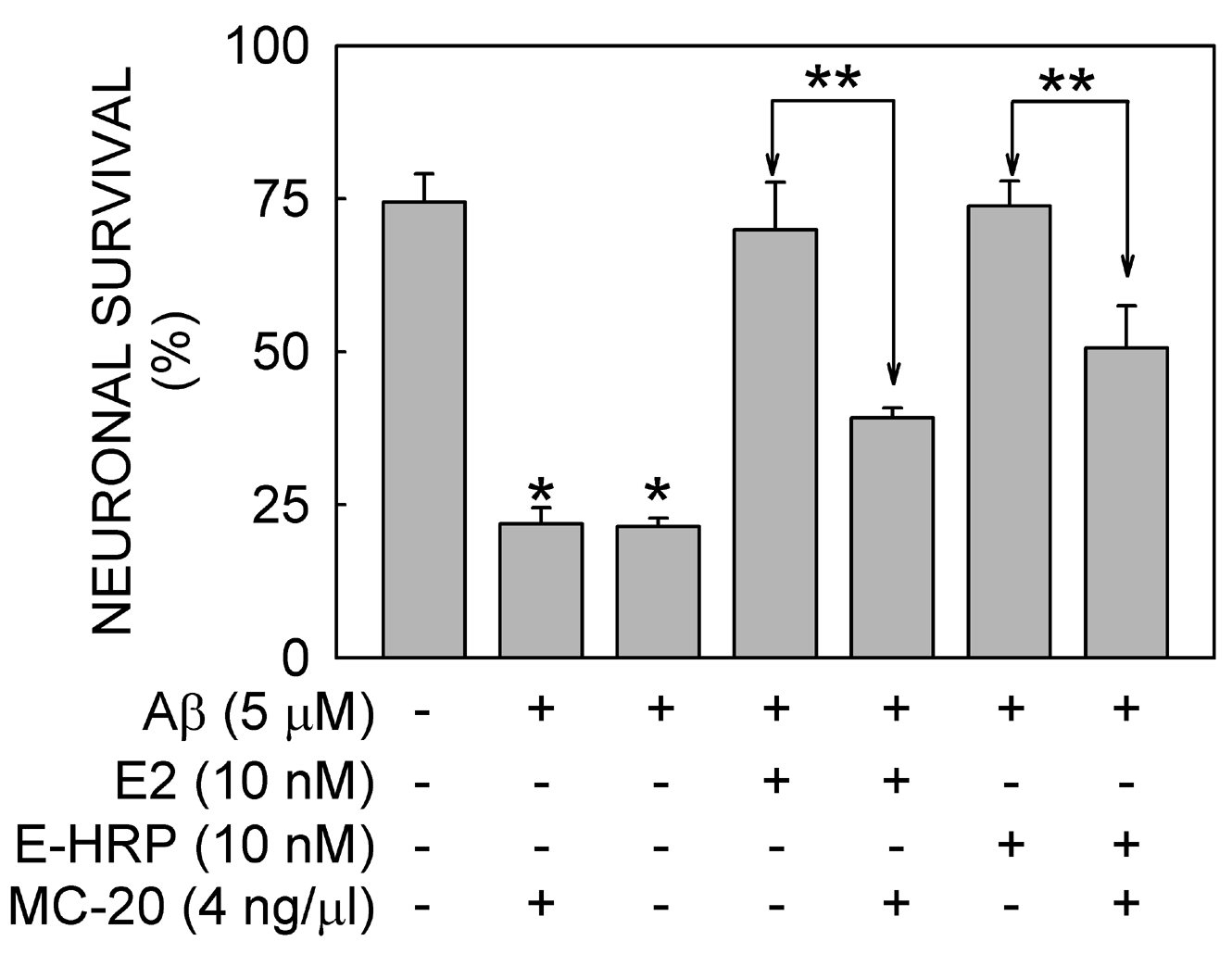
Using confocal microscopy on SN56 cells fixed under non-permeabilized conditions and exposed to a polyclonal antibody (MC-20) directed to ERα, we have detected an ER at the plasma membrane level. Western blot analyses of purified cell membrane fractions revealed the presence of two forms of ER (67 and 80 kDa). Fifteen minutes exposure to either E2 or E-HRP prevented Aβ-induced cell death. This effect was reduced by both the ER antagonist ICI 182,780 or MC-20 antibody (see Fig. 1).
Using E-HRP and E2 coupled to BSA and a fluorescence probe (E-BSA-FITC), we found binding sites for E2 at the surface of SN56 cells. Binding of both conjugates was blocked by pre-treatment with either E2 or ICI 182,780. Pre-exposure to increasing concentrations of MC-20 antibody significantly decreased E-BSA-FITC labelling. These results suggest that the ER observed at the plasma membrane domain shares structural similarities with its intracellular counterpart, and that it might participate in membrane-mediated neuroprotective oestrogen actions in SN56 cells.
This work was supported by SAF2001-3614-C03-01/02.