The epithelial di- and tri-peptide transporter PepT1 couples substrate and proton movement in such a way that transport is powered both by the physiological proton gradient across the apical membrane and by the membrane potential (Temple et al. 1995). Previously, uptake of the neutral dipeptide D-Phe-L-Gln into R282E-PepT1-expressing oocytes at pHout 5.5 had revealed a functional transporter (Meredith, 2003), which has now been further characterised. Here we report that the site-directed mutagenesis of this arginine residue abolishes the proton coupling of peptide transport.
Arginine282 in rabbit PepT1 was mutated to a glutamate using a PCR-based protocol (Quikchange, Stratagene) and confirmed by DNA sequencing to produce R282E-PepT1. Uptake experiments into PepT1-expressing Xenopus laevis oocytes were performed as previously described (Meredith et al. 2000). Data are means ± S.E.M.
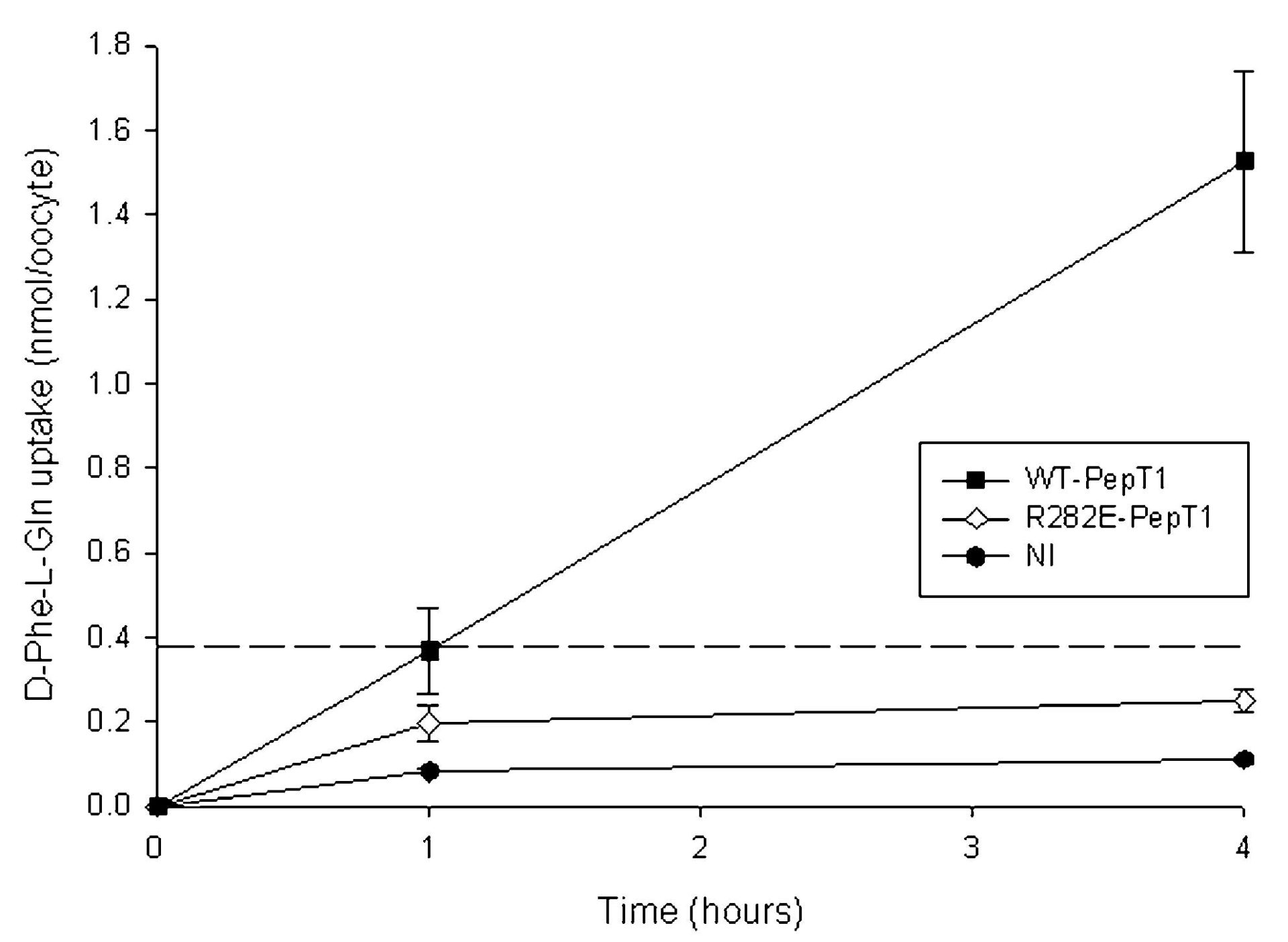
Uptake of D-Phe-L-Gln into oocytes expressing R282E-PepT1 was found to be unaffected by extracellular acidification (1.1 ± 0.4-fold increase, n = 2), unlike for the wild-type PepT1, which was stimulated by reducing pHout from 7.4 to 5.5 by 2.4 ± 0.3-fold (P < 0.05, Student’s paired t test, n = 4). To investigate whether this was due to peptide uptake no longer being coupled to the proton electrochemical gradient, the ability of the oocytes to perform concentrative uptake at pHout 5.5 was measured, as shown in Fig. 1. As can be seen, R282E-PepT1-expressing oocytes were not able to concentrate D-Phe-L-Gln, unlike those expressing WT-PepT1 which achieved approximately 4-fold concentration above the extracellular level after 4 h.
Taken together, the data that D-Phe-L-Gln uptake by R282E-PepT1 is not stimulated by external acidification nor can R282E-PepT1-expressing oocytes concentrate dipeptide above the external concentration strongly suggest that the proton and dipeptide translocation have been uncoupled, and that R282E-PepT1 behaves as a facilitated diffusion system.
This work is generously funded by the Wellcome Trust.