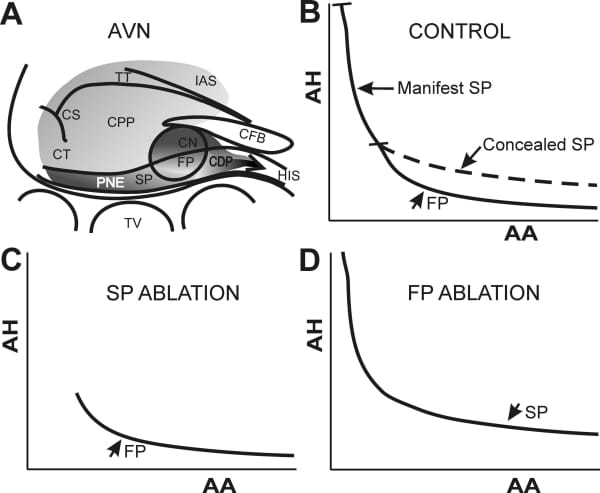
The rate-dependent properties of the AV node account for the filtering of atrial impulses during supraventricular tachyarrhythmias. These properties may generate important beat-to-beat changes in nodal refractory period and conduction time even in the absence of autonomic modulation. In addition, the AV node has rate-sensitive dual pathway properties that not only generate echo beats and reentrant rhythms but also contribute to normal conduction. The anatomical and functional substrate underlying AV nodal rate-dependent and dual pathway function remains ill-defined. While the compact node (Figure 1A) is considered a pivotal structure in rate-dependent function, nodal inputs are often considered to play a key role in dual pathways. Several hypotheses have been put forward to account for dual pathway function. Initially, a functional asymmetry between the crista terminalis and interatrial septum inputs extending to an unspecified portion of the compact node has been postulated. Some investigators have suggested that inputs alone could provide the substrate for two or more pathways. In contrast, others have concluded that the compact node is the primary site of dual pathways. Alternately, the compact node may act as the slow pathway whereas a bypass track made of transitional tissues acts as the fast pathway. Our own histological and functional data indicate that the posterior extension and compact node provide the substrate for slow and fast pathway conduction, respectively. Our current goal is to provide a unified hypothesis for AV nodal rate-dependent and dual pathway function. More specifically, we hypothesize that overall rate-dependent AV nodal properties reflect the lumped expression of the rate-dependent properties displayed by the posterior extension (slow pathway) and compact node (fast pathway). According to this scheme, nodal inputs serve as a common proximal pathway whereas the lower nodal bundle serves as a common distal pathway. A common proximal pathway implies that fast or slow pathway conduction can result from any input activation pattern. A common distal pathway implies that its activation can result from either slow or fast pathway conduction. Our data also imply that a slow pathway conduction proceeds directly from the posterior extension to the lower nodal bundle i.e., bypasses the compact node. Conversely, fast pathway conduction from the compact node to the lower nodal bundle bypasses the posterior extension. In support of our hypothesis is the finding that nodal inputs are not functionally asymmetric enough to account for dual pathways. In addition, rate-dependence of nodal inputs is trivial. Our data also provide direct evidence for the involvement of the posterior extension and compact node in slow and fast pathway, respectively. Fast pathway conduction prevails in long and intermediate cycle length ranges whereas slow pathway conduction takes place in short cycle length range because of the long refractory period of the fast pathway (Figure 1B). When fast pathway conduction prevails, slow pathway conduction is concealed. Ablation microlesions targeting the posterior extension amputate the left steep portion of the nodal recovery curve but leave intact the flat portion of the curve corresponding to the fast pathway (Figure 1C). When microlesions are applied at the junction between transitional and compact node tissues, nodal conduction time is not altered over short cycle length range (Figure 1D). That ablation reveals otherwise concealed slow pathway conduction as prolonged nodal conduction times over intermediate and long cycle lengths. Microlesions applied at both sites cause a complete AV block. Taken together, the above data indicate that the overall nodal recovery curve is a composite of recovery properties of slow and fast pathway, each accounting for a specific portion of the curve. Preliminary data show that, except for an upward shift, the recovery curves of slow, fast and dual pathways were not otherwise altered by fatigue. Therefore, overall fatigue may also be explained by individual effects of rate on slow and fast pathway. In conclusion, overall rate-dependent AV nodal function arises from the individual properties of the slow and fast pathway. Consequently, AV nodal rate-dependent and dual pathway properties can be reconciled and lumped into a single functional scheme.
University of Manchester (2007) Proc Physiol Soc 8, SA10
Research Symposium: AVN function: one node two pathways
J. Billette1, M. Lavallée1
1. Physiology, Université de Montréal, Montreal, QC, Canada.
View other abstracts by:
Figure 1: Schematic representation of dual AV nodal pathways. A, AV node structures and landmarks. B, dual pathway conduction curve (normal AV node). C, FP conduction curve after posterior extension ablation. D, SP conduction curve after FP ablation. FP, fast pathway. SP, slow pathway. CPP, common proximal pathway. CDP, common distal pathway. AH, atrial-His interval. AA, atrial cycle length.
Where applicable, experiments conform with Society ethical requirements.