Burke R, Rudomin P & Zajac F (1976). Brain Res 109, 515-529.
University of Heidelberg (2006) Proc Physiol Soc 4, PC10
Poster Communications: Bjørn G. Nielsen1
1. Department of Physics, Technical University of Denmark, Kgs. Lyngby, Denmark.
In response to a regular spike-train, skeletal muscles will gradually increase their tension until a plateau is reached that matches the spike-train frequency. This is a relatively slow process taking in the order of 50-500 ms depending on muscle type and activation frequency (see e.g. Burke et al. 1976), and it is an indication that force production is a cumulative process that depends on systems with slower dynamics than the spikes themselves. In experiments (Abbate et al. 2001 and references therein) the actual position of the force-plateau matching a particular frequency can be shifted by tetanic bursts of high-frequency stimulation in a process known as post-tetanic potentiation (PTP). It is generally accepted that the force increase associated with PTP is related to phosphorylation of myosin light chains (reviewed in Abbate et al. 2001); however, the phosphorylation itself might be triggered by an increase of myoplasmic Ca2+ following tetanic stimulation of a muscle fibre (Decostre et al. 2000). A Ca2+ resource allocation based model of the excitation-contraction coupling has here been developed to investigate frequency-dependent changes in muscle force response. This model rests on the common assumption that Ca2+ resources are unevenly distributed throughout the muscle fibres (myoplasm, sarcoplasm and T-tubules). For simplicity it is also assumed that regulation and transport within and between these compartments follows simple first-order dynamics associated to protein actions either in the form of Ca2+ binding proteins (parvalbumin, calsequestrin and troponin C), or through the action of membrane bound Ca2+ channels (sarcoendoplasmatic reticulum Ca2+-ATPase, ryanodine receptor, dihydropyridine receptor). Each of these systems contributes to the overall Ca2+ dynamics via time constants ultimately determining the rate of Ca2+ transport between compartments. The system is highly sensitive to motoneuronal spike arrival times (triggering release of Ca2+ from sarcoplasm) and therefore several frequency-dependent muscle force responses like PTP and the catch-like effect of skeletal muscle (Burke et al. 1976) are easily modelled. It is concluded that the small amount of additional extracellular Ca2+ that gains access to the myoplasm via delayed slow opening of the L-type DHPR Ca2+ channel (Shtifman et al. 2004 and references therein) might be sufficient to account for PTP in response to tetanic activation. This would probably involve retrograde coupling in which RyR channels modulate currents through the DHPR channel (see review by Dulhunty et al. 2002).
View other abstracts by:
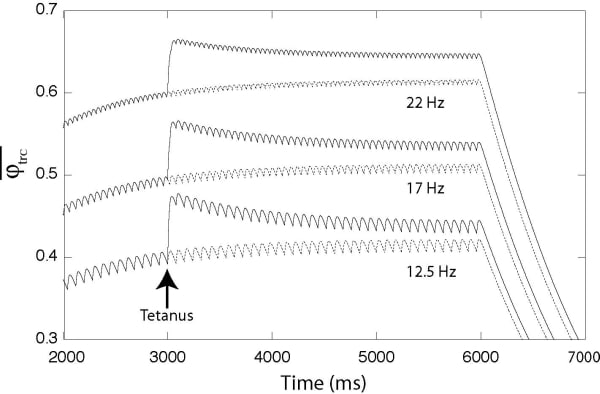
Figure 1. Fraction of Ca2+ bound to troponin C as a function of time during regular spiking activity (at 12.5 17 and 22 Hz). Arrow marks time at which the total amount of Ca2+ within the muscle fibre is suddenly increased by 5% here corresponding to cumulative additional extracellular Ca2+ entering the myoplasm during tetanus via slow L-type Ca2+ channels like the DHPR receptor.
Where applicable, experiments conform with Society ethical requirements.