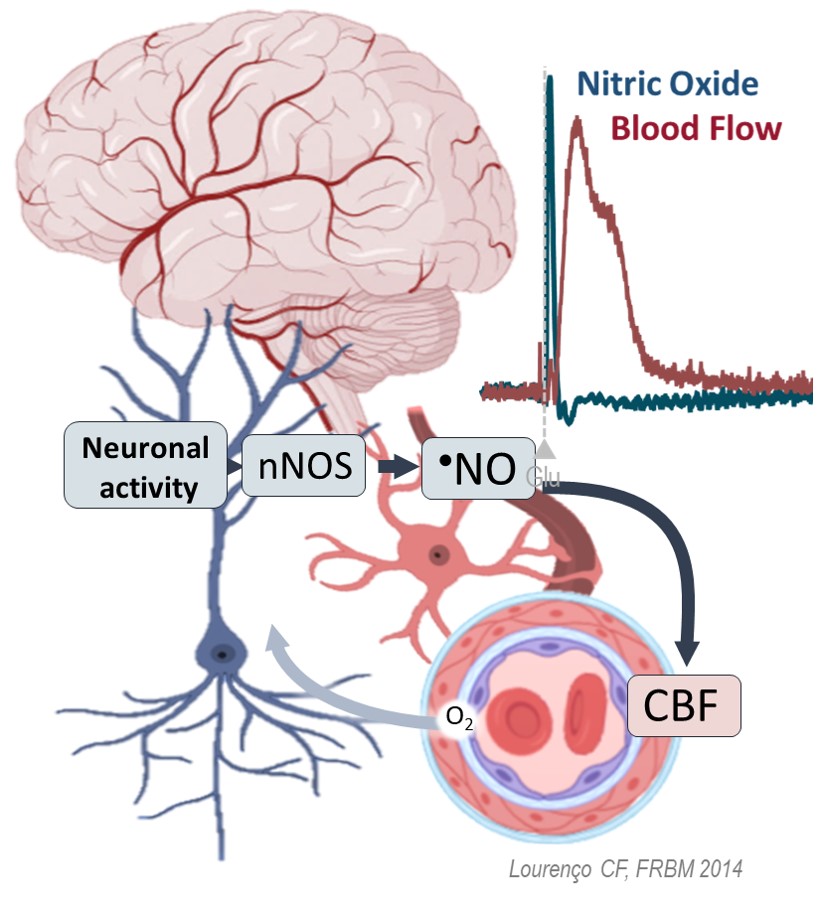
The brain´s function and structural integrity rely on a tightly regulated delivery of metabolic substrates (glucose and oxygen) matching the ongoing neuronal activity. This process – neurovascular coupling (NVC) – is critically orchestrated by nitric oxide (•NO) via the glutamate NMDAr-nNOS-sGC pathway, especially in the hippocampus, a brain region involved in memory processing (Lourenço et al., 2014, Figure 1). The dysfunction of NVC, linked to compromised •NO bioavailability and bioactivity, has been increasingly associated with neuronal dysfunction in several neurodegenerative conditions, such as Alzheimer’s Disease and Vascular Cognitive Impairment and Dementia (VCID), being recognized as a relevant contributor to dysfunctional cascade leading to neurodegeneration and cognitive decline. In addition to the canonical enzymatic pathways, •NO can be produced upon the sequential reduction in vivo along the nitrate-nitrite-NO pathway. In this line, we hypothesized that dietary nitrate can be used as a strategy to foster •NO-dependent NVC under conditions of limited •NO bioavailability.
We tested our hypothesis in two rodent models mimicking specific features of VCID: 1) 2VO rats modeling cerebral hypoperfusion and 2) diabetic Goto-Kakizaki (GK) rats modeling microvascular dysfunction. Dietary nitrate intervention was achieved by providing sodium nitrate in water ad libitum for 8-12 weeks. The NVC functionality was accessed by measuring hemodynamic responses to glutamatergic activation in the hippocampus in vivo by laser Doppler flowmetry simultaneously with coupled •NO dynamics by electrochemical methods. The spatial working and reference memory dependent on hippocampal function was assessed in the Barnes maze paradigm. NADPH oxidase-mediated superoxide formation was detected by a lucigenin-dependent chemiluminescence assay. All the procedures were performed in compliance with the ethical regulations for animal-based research.
We found a compromised NVC in response to glutamatergic activation in both animal models (CBF changes were reduced to 41±3% in 2VO and 33±4% in GK as compared to their controls), which in GK rats were coupled with •NO transients with a shorter and faster profile. Of notice, in both GK and 2VO rats, these findings were coupled to a compromised spatial memory performance. As hypothesized, the intervention with dietary nitrate was able to counteract the spatial memory decline in both animal models which was correlated with an improvement in the NVC. Also, dietary nitrate reduced the NADPH activity in the hippocampus of both 2VO and GK rats.
Overall data support a close mechanistic association between hippocampal neuronal-triggered •NO concentration dynamics, hemodynamic responses, and cognitive performance, establishing the functionality of NVC as a critical early factor to consider in the cascade of events leading to cognitive decline in VCID that can be improved by dietary nitrate intervention.