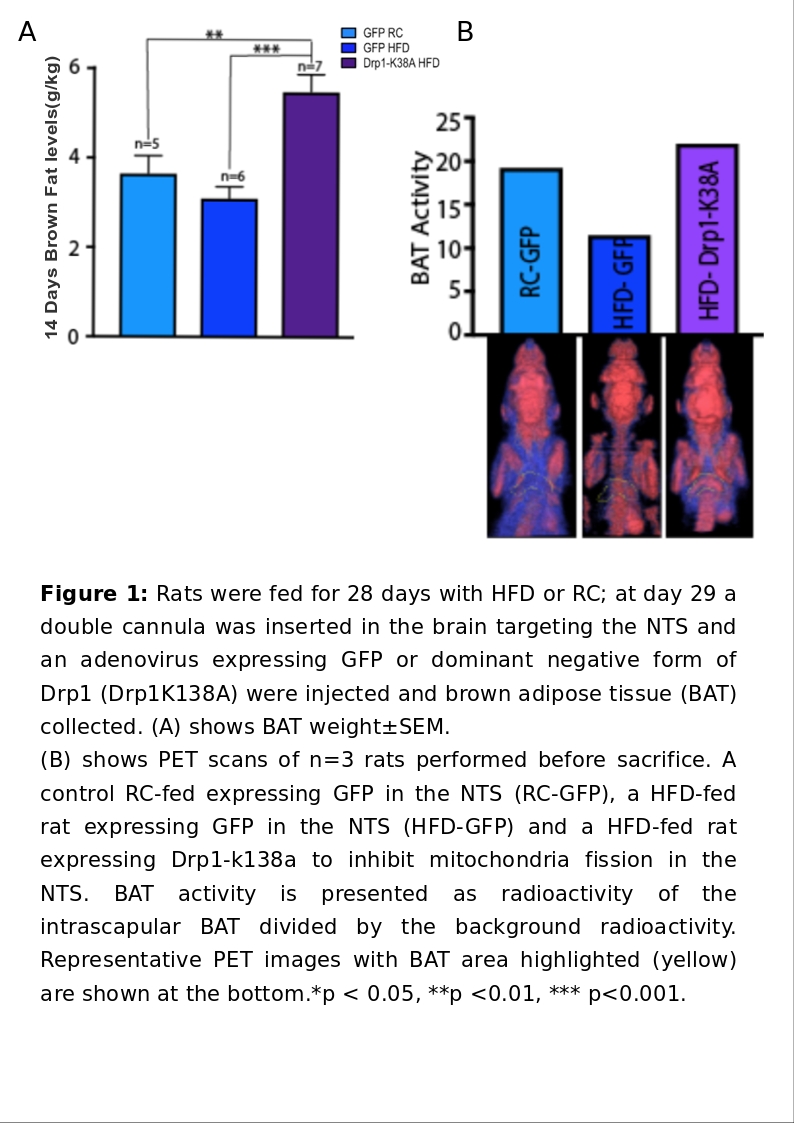
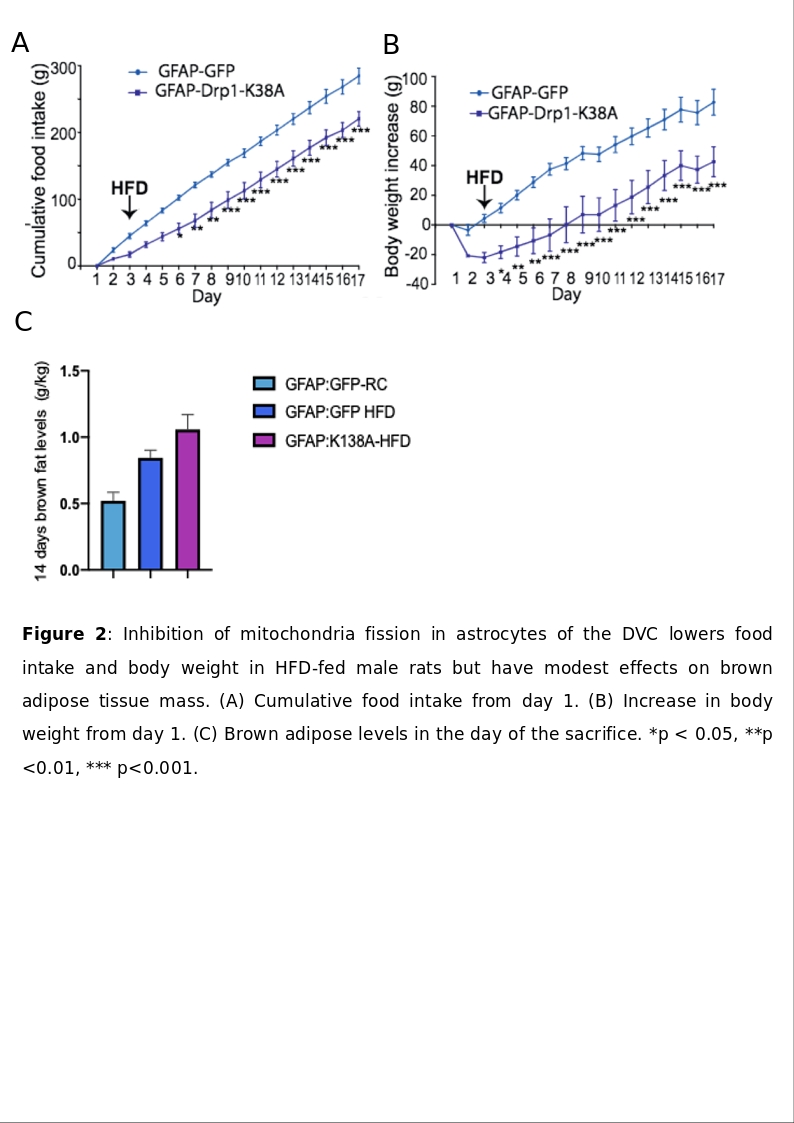
Obesity epidemic is rising globally posing a burden on healthcare systems worldwide, and behavioural interventions may not be sufficient to tackle obesity. The recent discovery of brown adipose tissue (BAT) in humans, has been shown to positively regulate energy expenditure, raising new possibilities. BAT activation is driven by the central nervous system (CNS); the Nucleus of the Tractus Solitarius (NTS) in the Dorsovagal complex (DVC) receives information on nutritional status from visceral vagal afferents. An increase in glutamatergic vagal inputs to the NTS, inhibiting the physiological cooling-activation of BAT, exist in high-fat diet (HFD) fed rats (Madden&Morrison, 2016). Moreover, studies have found that HFD causes an increase of mitochondria fission in the DVC, which is regulated by dynamin related protein 1(Drp1) (Filippi et al, 2017). Previous studies within our group have shown that injecting a dominant negative form of Drp1 (Drp1-K138A) to inhibit mitochondria fission in rats NTS decreased food intake, restored insulin sensitivity in the NTS and prevented weight gain in HFD fed-obese rats, while these effects are not seen in HFD-GFP expressing controls. Furthermore, HFD alters astrocytes functionality in the NTS (MacDonald et al, 2019), but the role of mitochondria dynamics is still unknown in this cellular-subset. We aim to investigate whether the aforementioned beneficial effects are due to a DVC-driven increase in BAT amount and function; We also aim to establish the cellular network involved in this pathway. An obese model (Yue et al, 2016) (1) and a non-obese model (2) of male Sprague-Dawley rats were anesthetised with 4% isoflurane by inhalation and submitted to stereotactic surgery to implant a bilateral cannula in the NTS (day 0). On day 1 animals were injected with an adenovirus expressing either Drp1K138A or GFAP:Drp1K138A or GFP or GFAP:GFP and kept for 14 days on either HFD(5.51kcal/g) or control diet (3.93kcal/g). On day 15 animals were culled with pentobarbital (60 mg/kg i.p) and BAT dissected and weighted. A pilot PET study (n=3, obese model) was performed before sacrifice. Where applicable, values are mean±SEM, analysed with ANOVA. (1) The amount of BAT in HFD-K138A (5.8±0.2g/kg) animals was 44% higher (p<.001) (n=9) than that of GFP-HFD (3.0±0.1g/kg) and 38%(p<0.01) higher than normal GFP-RC control rats(3.8±0.2g/kg)(n=9). The PET scan revealed that BAT activity level in HFD-K138A was 46% higher than that GFP-HFD and 16% higher than GFP-RC control. (2) GFAP:Drp1K138A administration produced a decrease in body weight and cumulative food intake in GFAP:Drp1K138A-HFD-fed rats(n=12) which become significant from day 2 and day 3 respectively, compared with GFAP:GFP-HFD expressing controls(n=12). A trend of increase in BAT mass in in GFAP:Drp1K138A-HFD animals was observed, but this was not significant (p=0.1669) when compared to GFAP:GFP-HFD controls (n=8 GFAP:Drp1K138A-HFD, n=9 in GFAP:GFP-HFD). Global inhibition of mitochondria dynamics in the NTS appears to act as BAT recruiter and stimulator. Astrocytes are important to maintain body weight and suppress food intake in HFD, but the effects on BAT recruitment appear modest. Molecular analysis is required to determine effects on BAT activation and functionality upon mutant Drp1 administration to the NTS.
Future Physiology 2020 (Virutal) (2020) Proc Physiol Soc 46, PC0010
Poster Communications: Can mitochondria dynamics in the dorsovagal complex modulate brown adipose tissue activation and recruitment to prevent an obese phenotype?
Arianna Fozzato1, Bianca Patel1, Lauryn New1, Beatrice Filippi1, Susan Deuchars1
1 University of Leeds, Leeds, United Kingdom
View other abstracts by:
Where applicable, experiments conform with Society ethical requirements.